Summary
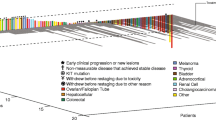
Combining different targeted anticancer agents may improve clinical outcomes. This Phase I study investigated cediranib, an oral inhibitor of vascular endothelial growth factor signalling in combination with saracatinib, an oral Src inhibitor. The primary endpoint was safety/tolerability. Secondary assessments included pharmacokinetics and preliminary efficacy. Patients and Methods Patients with advanced solid tumours received cediranib 20, 30 or 45 mg/day for 7 days followed by daily treatment with cediranib at the same dose plus saracatinib 175 mg/day. Results Thirty-nine patients received cediranib (20 mg, n = 6; 30 mg, n = 6; 45 mg, n = 27 [n = 20 in cohort expansion]) plus saracatinib. In the cediranib 45 mg cohort, 59% of patients required dose reduction/pause compared with 33% in each of the other two cohorts. There was one dose-limiting toxicity (hypertension; 45 mg cohort). The most common adverse events were hypertension (67%), diarrhoea (62%), dysphonia (46%) and fatigue (39%). There was no evidence of a clinically significant effect of saracatinib on cediranib pharmacokinetics and vice versa. 22/35 evaluable patients had a best response of stable disease. Conclusions All cediranib doses were tolerated; however, in patients with advanced solid tumours, for combination with saracatinib 175 mg/day, cediranib 20 or 30 mg/day was more sustainable than 45 mg/day.



Similar content being viewed by others
References
Rosen LS (2005) VEGF-targeted therapy: therapeutic potential and recent advances. Oncologist 10:382–391
Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jürgensmeier JM, Ogilvie DJ (2005) AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res 65:4389–4400
Drevs J, Siegert P, Medinger M, Mross K, Strecker R, Zirrgiebel U, Harder J, Blum H, Robertson J, Jürgensmeier JM, Puchalski TA, Young H, Saunders O, Unger C (2007) Phase I clinical study of AZD2171, an oral vascular endothelial growth factor signaling inhibitor, in patients with advanced solid tumors. J Clin Oncol 25:3045–3054
Yamamoto N, Tamura T, Yamamoto N, Yamada K, Yamada Y, Nokihara H, Fujiwara Y, Takahashi T, Murakami H, Boku N, Yamazaki K, Puchalski TA, Shin E (2009) Phase I, dose escalation and pharmacokinetic study of cediranib (RECENTIN), a highly potent and selective VEGFR signaling inhibitor, in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 64:1165–1172
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Matulonis UA, Berlin S, Ivy P, Tyburski K, Krasner C, Zarwan C, Berkenblit A, Campos S, Horowitz N, Cannistra SA, Lee H, Lee J, Roche M, Hill M, Whalen C, Sullivan L, Tran C, Humphreys BD, Penson RT (2009) Cediranib, an oral inhibitor of vascular endothelial growth factor receptor kinases, is an active drug in recurrent epithelial ovarian, fallopian tube, and peritoneal cancer. J Clin Oncol 27:5601–5606
Hyams DM, de Oliveira C, Snyder R, Klein P, Vinholes J, Audeh MW, Alencar V, Lombard J, Mookerjee B, Xu J, Chan A (2009) Cediranib in combination with fulvestrant in hormone-sensitive metastatic breast cancer: a phase II randomized study. Cancer Res 69(24 Suppl 3):abst 204
Saura C, Baselga J, Herbst R, del Campo J, Marotti M, Tessier J, Collins B, Heymach J (2009) Antitumor activity of cediranib in patients with metastatic or recurrent head and neck cancer (HNC) or recurrent non-small cell lung cancer (NSCLC): an open-label exploratory study. J Clin Oncol 27(15S):abst 6023
Garland LL, Chansky K, Wozniak A, Tsao A, Gadgeel S, Vershraegen C, Da Silva M, Redman M, Gandara D (2009) SWOG S0509: a phase II study of novel oral antiangiogenic agent AZD2171 (NSC-732208) in malignant pleural mesothelioma. J Clin Oncol 27(15S):abst 7511
Gardner K, Judson I, Leahy M, Barquin E, Marotti M, Collins B, Young H, Scurr M (2009) Activity of cediranib, a highly potent and selective VEGF signaling inhibitor, in alveolar soft part sarcoma. J Clin Oncol 27(15S):abst 10523
Gardner K, Judson I, Leahy M, Barquin E, Marotti M, Collins B, Young H, Scurr M (2009) A phase II study of cediranib in patients with metastatic gastrointestinal stromal tumours (GIST) and metastatic soft tissue sarcoma (STS) (including alveolar soft part sarcoma [ASPS]). European J Cancer Suppl 7:591
Mulders P, Hawkins R, Nathan P, de Jong I, Osanto S, Porfiri E, Protheroe A, Mookerjee B, Pike L, Gore ME (2009) Final results of a phase II randomised study of cediranib (RECENTIN™) in patients with advanced renal cell carcinoma (RCC). Eur J Cancer Suppl 7(3):21 (abst 49LBA)
Laurie SA, Gauthier I, Arnold A, Shepherd FA, Ellis PM, Chen E, Goss G, Powers J, Walsh W, Tu D, Robertson J, Puchalski TA, Seymour L (2008) Phase I and pharmacokinetic study of daily oral AZD2171, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with carboplatin and paclitaxel in patients with advanced non-small-cell lung cancer: the national cancer institute of Canada clinical trials group. J Clin Oncol 26:1871–1878
Chen E, Jonker D, Gauthier I, Maclean M, Wells J, Powers J, Seymour L (2009) Phase I study of cediranib in combination with oxaliplatin and infusional 5-fluorouracil in patients with advanced colorectal cancer. Clin Cancer Res 15:1481–1486
Goss G, Shepherd FA, Laurie S, Gauthier I, Leighl N, Chen E, Feld R, Powers J, Seymour L (2009) A phase I and pharmacokinetic study of daily oral cediranib, an inhibitor of vascular endothelial growth factor tyrosine kinases, in combination with cisplatin and gemcitabine in patients with advanced non-small cell lung cancer: a study of the national cancer institute of Canada clinical trials group. Eur J Cancer 45:782–788
Heymach JV, Glisson B, Doebele RC, Huang C, Gandara D, Le Scouiller S, Marotti M, Camidge DR (2010) Phase I open-label study of cediranib plus etoposide (E) and cisplatin (P) as first-line therapy for patients (pts) with small cell lung cancer (SCLC) or lung neuroendocrine cancer (NEC). J Clin Oncol 28(15S):abst 7050
Aligayer H, Boyd DD, Heiss MM, Abdalla EK, Curley SA, Gallick GE (2002) Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer 94:344–351
Bild AH, Parker JS, Gustafson AM, Acharya CR, Hoadley KA, Anders C, Marcom PK, Carey LA, Potti A, Nevins JR, Perou CM (2009) An integration of complementary strategies for gene-expression analysis to reveal novel therapeutic opportunities for breast cancer. Breast Cancer Res 11:R55
Irby RB, Yeatman TJ (2000) Role of Src expression and activation in human cancer. Oncogene 19:5636–5642
Wheeler DL, Iida M, Dunn EF (2009) The role of Src in solid tumors. Oncologist 14:667–678
Green TP, Fennell M, Whittaker R, Curwen J, Jacobs V, Allen J, Logie A, Hargreaves J, Hickinson DM, Wilkinson RW, Elvin P, Boyer B, Carragher N, Ple PA, Bermingham A, Holdgate GA, Ward WH, Hennequin LF, Davies BR, Costello GF (2009) Preclinical anticancer activity of the potent, oral Src inhibitor AZD0530. Mol Oncol 3:248–261
Hennequin LF, Allen J, Breed J, Curwen J, Fennell M, Green TP, der Lambert-van BC, Morgentin R, Norman RA, Olivier A, Otterbein L, Ple PA, Warin N, Costello G (2006) N-(5-chloro-1,3-benzodioxol-4-yl)-7-[2-(4-methylpiperazin-1-yl)ethoxy]-5- (tetrahydro-2H-pyran-4-yloxy)quinazolin-4-amine, a novel, highly selective, orally available, dual-specific c-Src/Abl kinase inhibitor. J Med Chem 49:6465–6488
Helfrich BA, Conner A, Conageski C, Behbakht K, Spillman (2010) Anti-proliferative activity of c-src kinase inhibitor saracatinib correlates with IGF1R and EGFR expression in ovarian cancer cell lines. Gynecol Oncol 116:abst 412
Baselga J, Cervantes A, Martinelli E, Chirivella I, Hoekman K, Hurwitz HI, Jodrell DI, Hamberg P, Casado E, Elvin P, Swaisland A, Iacona R, Tabernero J (2010) Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin Cancer Res 16:4876–4883
Hannon RA, Clack G, Rimmer M, Swaisland A, Lockton JA, Finkelman RD, Eastell R (2009) Effects of the Src kinase inhibitor saracatinib (AZD0530) on bone turnover in healthy men: a randomized, double-blind, placebo-controlled, multiple ascending dose phase I trial. J Bone Miner Res 25:463–471
Edwards J (2010) Src kinase inhibitors: an emerging therapeutic treatment option for prostate cancer. Expert Opin Investig Drugs 19:605–614
Han LY, Aamdal S, Bowen E, Freyer G, Kristensen G, Jones R, Pujade-Lauraine E, De Vries L, Prahladan M, Kaye SB (2010) Treatment profile of saracatinib (AZD0530) in combination with chemotherapy in patients with advanced ovarian carcinoma (OC). Gynecol Oncol 116:abst 151
Weis S, Cui J, Barnes L, Cheresh D (2004) Endothelial barrier disruption by VEGF-mediated Src activity potentiates tumor cell extravasation and metastasis. J Cell Biol 167:223–229
Jürgensmeier JM, Kendrew J, Odedra R, Logie A, Wood P, Valentine P, Barnett S, Wilkinson RW, Ogilvie DJ, Elvin P, Smith P, Ryan A, Wedge SR (2010) Cediranib alone and in combination with mechanistically distinct antitumor therapies in vivo. Proc Am Assoc Cancer Res 51:abst 1372
Ryan CJ, Stadler WM, Roth B, Hutcheon D, Conry S, Puchalski T, Morris C, Small EJ (2007) Phase I dose escalation and pharmacokinetic study of AZD2171, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinase, in patients with hormone refractory prostate cancer (HRPC). Invest New Drugs 25:445–451
Langenberg MH, van Herpen CM, de Bono J, Schellens JH, Unger C, Hoekman K, Blum HE, Fiedler W, Drevs J, Le Maulf F, Fielding A, Robertson J, Voest EE (2009) Effective strategies for management of hypertension after vascular endothelial growth factor signaling inhibition therapy: results from a phase II randomized, factorial, double-blind study of cediranib in patients with advanced solid tumors. J Clin Oncol 27:6152–6159
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205–216
Goss GD, Arnold A, Shepherd FA, Dediu M, Ciuleanu TE, Fenton D, Zukin M, Walde D, Laberge F, Vincent MD, Ellis PM, Laurie SA, Ding K, Frymire E, Gauthier I, Leighl NB, Ho C, Noble J, Lee CW, Seymour L (2010) Randomized, double-blind trial of carboplatin and paclitaxel with either daily oral cediranib or placebo in advanced non-small-cell lung cancer: NCIC clinical trials group BR24 study. J Clin Oncol 28:49–55
Acknowledgements
We thank Dr Helen Jones, from Mudskipper Bioscience, who provided writing support funded by AstraZeneca. This study was supported by funding from AstraZeneca.
Conflicts of interest
W.E.E.E has received lecturer fees and compensation as an advisory role (AstraZeneca, Roche, GSK, Novartis, Pfizer, Sanofi-aventis, Bayer Schering, Boehringer Ingelheim, Bristol Myers, OSI, Merck Serono, Amgen). S.LS., M.M. and K.H.B. are employees of AstraZeneca. T.T., B.S., T.C.G., V.S., D.S. and J.D. have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trarbach, T., Schultheis, B., Gauler, T.C. et al. Phase I open-label study of cediranib, an oral inhibitor of VEGF signalling, in combination with the oral Src inhibitor saracatinib in patients with advanced solid tumours. Invest New Drugs 30, 1962–1971 (2012). https://doi.org/10.1007/s10637-011-9754-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9754-x