Summary
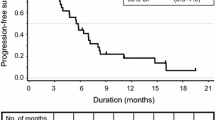
Background Treatment options for metastatic colorectal cancer (CRC) are limited after a fluoropyrimidine, oxaliplatin and irinotecan; novel agents need to be explored in this setting. Dasatinib, an oral inhibitor of Src family kinases, inhibits proliferation in CRC cell lines and has antitumor activity in CRC xenograft models. Patients and methods We conducted a multi-center phase II trial of dasatinib in unresectable, previously-treated metastatic CRC patients. No more than 2 prior chemotherapy regimens were permitted, which must have contained a fluoropyrimidine, oxaliplatin and irinotecan. The primary endpoint was progression-free survival (PFS) at 4 months. The Simon two-stage design required that at least 5 of the first 19 patients be progression-free at 4 months to expand to a second stage. Results Nineteen patients enrolled at 9 centers. The study was terminated after the first stage due to lack of efficacy. There were no objective responses; 1 patient (5%) had stable disease for 7.3 months. The PFS rate at 4 months was 5.3% (90% CI: 0.3, 22.6). Median PFS was 1.6 months (90% CI: 1.4, 1.8). Median overall survival was 5.1 months (90% CI: 2.4, 6.3). Grade 3/4 toxicities included fatigue in 16% of patients, and anemia, anorexia, nausea/vomiting and dyspnea in 11%. Conclusion Dasatinib is inactive as a single agent in previously treated metastatic CRC patients.


Similar content being viewed by others
References
Van Cutsem E, Peeters M, Siena S et al (2007) Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol 25(13):1658–1664
Amado RG, Wolf M, Peeters M et al (2008) Wild-type KRAS is required for panitumumab efficacy in patients with metastatic colorectal cancer. J Clin Oncol 26(10):1626–1634
Dahabreh IJ, Terasawa T, Castaldi PJ, Trikalinos TA (2011) Systematic review: anti-epidermal growth factor receptor treatment effect modification by KRAS mutations in advanced colorectal cancer. Ann Intern Med 154(1):37–49
Mayer EL, Krop IE (2010) Advances in targeting SRC in the treatment of breast cancer and other solid malignancies. Clin Cancer Res 16(14):3526–3532
Windham TC, Parikh NU, Siwak DR et al (2002) Src activation regulates anoikis in human colon tumor cell lines. Oncogene 21(51):7797–7807
Talamonti MS, Roh MS, Curley SA, Gallick GE (1993) Increase in activity and level of pp 60c-src in progressive stages of human colorectal cancer. J Clin Invest 91(1):53–60
Aligayer H, Boyd DD, Heiss MM et al (2002) Activation of Src kinase in primary colorectal carcinoma: an indicator of poor clinical prognosis. Cancer 94(2):344–351
Staley CA, Parikh NU, Gallick GE (1997) Decreased tumorigenicity of a human colon adenocarcinoma cell line by an antisense expression vector specific for c-Src. Cell Growth Differ 8(3):269–274
Ellis LM, Staley CA, Liu W et al (1998) Down-regulation of vascular endothelial growth factor in a human colon carcinoma cell line transfected with an antisense expression vector specific for c-src. J Biol Chem 273(2):1052–1057
Golas JM, Lucas J, Etienne C et al (2005) SKI-606, a Src/Abl inhibitor with in vivo activity in colon tumor xenograft models. Cancer Res 65(12):5358–5364
Dasatinib Investigator’s Brochure (2006)
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92(3):205–216
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10(1):1–10
Kaplan E, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38:29–41
Miller AA, Pang H, Hodgson L et al (2010) A phase II study of dasatinib in patients with chemosensitive relapsed small cell lung cancer (Cancer and Leukemia Group B 30602). J Thorac Oncol 5(3):380–384
Brooks HD, Glisson BS, Bekele BN et al (2010) Phase 2 study of dasatinib in the treatment of head and neck squamous cell carcinoma. Cancer [Epub ahead of print]
Kopetz S, Lesslie DP, Dallas NA et al (2009) Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res 69(9):3842–3849
Nautiyal J, Banerjee S, Kanwar SS et al (2011) Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int J Cancer 128(4):951–961
Lieu CH, Wolff RA, Eng C et al (2010) Phase IB study of the Src inhibitor dasatinib with FOLFOX and cetuximab in metastatic colorectal cancer. J Clin Oncol 28:3536
http://www.clinicaltrials.gov/ct2/show/NCT00501410?term=dasatinib+FOLFOX+cetuximab&rank=1 (accessed January 17, 2011)
Demetri GD, Lo Russo P, MacPherson IR et al (2009) Phase I dose-escalation and pharmacokinetic study of dasatinib in patients with advanced solid tumors. Clin Cancer Res 15(19):6232–6240
Johnson FM, Agrawal S, Burris H et al (2010) Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer 116(6):1582–1591
Shah NP, Kantarjian HM, Kim DW et al (2008) Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol 26(19):3204–3212
Funding source
NCI Grant N01-CM-62201.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, M.R., Wroblewski, K., Polite, B.N. et al. Dasatinib in previously treated metastatic colorectal cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest New Drugs 30, 1211–1215 (2012). https://doi.org/10.1007/s10637-011-9681-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9681-x