Summary
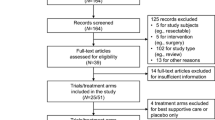
Progression-free survival (PFS) and time to progression (TTP) have been reported to correlate with overall survival (OS) in several types of cancers. To our knowledge, however, their use in the evaluation of new agents for AGC has not been investigated. We evaluated the potential of PFS and TTP to act as surrogates of OS in clinical trial settings. Randomized trials of systemic chemotherapy for advanced gastric cancer were identified by comprehensive electronic and manual search. Correlations between PFS/TTP and OS were evaluated. Thirty-six trials with a total of 83 treatment arms and 10,484 patients were selected for analysis. The nonparametric Spearman rank correlation coefficient (ρ) between median PFS/TTP and OS was 0.70 (95% CI, 0.59 to 0.82) and the correlation coefficient between hazard ratios in PFS/TTP and OS was 0.80 (95% CI, 0.68 to 0.92). Correlation tended to be higher in trials reporting PFS (ρ = 0.85; 0.72–0.97) than in those reporting TTP (ρ = 0.60; 0.24–0.97), trials in Non-Asian countries (ρ = 0.80; 0.61–0.99) than Asia (ρ = 0.67; 0.39–0.94), trials in patients with measurable lesions only (ρ = 0.91; 0.77–1.00) than in those including non-measurable lesions (ρ = 0.71; 0.50–0.93), albeit that none of these differences was significant. Our results indicate that improvements in PFS/TTP in advanced gastric cancer strongly correlate with improvements in OS. Further research is needed to clarify the surrogacy of PFS/TTP for OS or the role of PFS as the true end point in future randomized clinical trials of chemotherapy for AGC.






Similar content being viewed by others
References
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24:2137–2150
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C et al (2006) Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 24:4991–4997
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F et al (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358:36–46
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M et al (2008) S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol 9:215–221
Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J et al (2009) Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 20:666–673
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V et al (2010) Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol 28:1547–1553
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A et al (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376:687–697
Thuss-Patience PC, Kretzschmar A, Deist T, Hinke A, Bichev D, Lebedinzew B et al (2009) Irinotecan versus best supportive care (BSC) as second-line therapy in gastric cancer: a randomized phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). J Clin Oncol 27:15s (suppl; abstr 4540)
Takiuchi H, Fukuda H, Boku N, Shimada Y, Nasu J, Hamamoto Y et al (2010) Randomized phase II study of best-available 5-fluorouracil (5-FU) versus weekly paclitaxel in gastric cancer (GC) with peritoneal metastasis (PM) refractory to 5-FU-containing regimens (JCOG0407). J Clin Oncol 28:15s (suppl; abstr 4052)
Takashima A, Boku N, Kato K, Mizusawa J, Nakamura K, Fukuda H et al (2010) Survival prolongation after treatment failure in patients with advanced gastric cancer (AGC): results from combined analysis of JCOG9205 and JCOG9912. J Clin Oncol 28:15s (suppl; abstr 4061)
Broglio KR, Berry DA (2009) Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst 101:1642–1649
Johnson KR, Ringland C, Stokes BJ, Anthony DM, Freemantle N, Irs A et al (2006) Response rate or time to progression as predictors of survival in trials of metastatic colorectal cancer or non-small-cell lung cancer: a meta-analysis. Lancet Oncol 7:741–746
Tang PA, Bentzen SM, Chen EX, Siu LL (2007) Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J Clin Oncol 25:4562–4568
Hotta K, Fujiwara Y, Matsuo K, Kiura K, Takigawa N, Tabata M et al (2009) Time to progression as a surrogate marker for overall survival in patients with advanced non-small cell lung cancer. J Thorac Oncol 4:311–317
Hackshaw A, Knight A, Barrett-Lee P, Leonard R (2005) Surrogate markers and survival in women receiving first-line combination anthracycline chemotherapy for advanced breast cancer. Br J Cancer 93:1215–1221
Burzykowski T, Buyse M, Piccart-Gebhart MJ, Sledge G, Carmichael J, Lück HJ et al (2008) Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J Clin Oncol 26:1987–1992
Saad ED, Katz A, Hoff PM, Buyse M (2010) Progression-free survival as surrogate and as true end point: insights from the breast and colorectal cancer literature. Ann Oncol 21:7–12
Hopewell S, Clarke M, Moher D, Wager E, Middleton P, Altman DG (2008) CONSORT for reporting randomised trials in journal and conference abstracts. Lancet 371:281–283
Parmar MB, Torri V, Stewart L (1998) Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med 17:2815–2834
Efron B (1979) Bootstrap methods: another look at the jackknife. Ann Stat 7:1–26
Cullinan SA, Moertel CG, Fleming TR, Rubin JR, Krook JE, Everson LK et al (1985) A comparison of three chemotherapeutic regimens in the treatment of advanced pancreatic and gastric carcinoma: fluorouracil vs fluorouracil and doxorubicin vs fluorouracil, doxorubicin, and mitomycin. JAMA 253:2061–2067
Kim NK, Park YS, Heo DS, Suh C, Kim SY, Park KC et al (1993) A phase III randomized study of 5-fluorouracil and cisplatin versus 5-fluorouracil, doxorubicin, and mitomycin C versus 5-fluorouracil alone in the treatment of advanced gastric cancer. Cancer 71:3813–3818
Cullinan SA, Moertel CG, Wieand HS, O’Connell MJ, Poon MA, Krook JE et al (1994) Controlled evaluation of three drug combination regimens versus fluorouracil alone for the therapy of advanced gastric cancer. North Central Cancer Treatment Group. J Clin Oncol 12:412–416
Loehrer PJ Sr, Harry D, Chlebowski RT (1994) 5-fluorouracil vs. epirubicin vs. 5-fluorouracil plus epirubicin in advanced gastric carcinoma. Invest New Drugs 12:57–63
Pyrhonen S, Kuitunen T, Nyandoto P, Kouri M (1995) Randomised comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 71:587–591
Kondo K, Sakamoto J, Nakazato H, Koike A, Kitoh T, Hachisuka K et al (2000) A phase III randomized study comparing doxifluridine and 5-fluorouracil as supportive chemotherapy in advanced and recurrent gastric cancer. Oncol Rep 7:485–490
Vanhoefer U, Rougier P, Wilke H, Ducreux MP, Lacave AJ, Van Cutsem E et al (2000) Final results of a randomized phase III trial of sequential high-dose methotrexate, fluorouracil, and doxorubicin versus etoposide, leucovorin, and fluorouracil versus infusional fluorouracil and cisplatin in advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group. J Clin Oncol 18:2648–2657
Ohtsu A, Shimada Y, Shirao K, Boku N, Hyodo I, Saito H et al (2003) Randomized phase III trial of fluorouracil alone versus fluorouracil plus cisplatin versus uracil and tegafur plus mitomycin in patients with unresectable, advanced gastric cancer: the Japan Clinical Oncology Group Study (JCOG9205). J Clin Oncol 21:54–59
Ross P, Nicolson M, Cunningham D, Valle J, Seymour M, Harper P et al (2002) Prospective randomized trial comparing mitomycin, cisplatin, and protracted venous-infusion fluorouracil (PVI 5-FU) with epirubicin, cisplatin, and PVI 5-FU in advanced esophagogastric cancer. J Clin Oncol 20:1996–2004
Tebbutt NC, Norman A, Cunningham D, Iveson T, Seymour M, Hickish T et al (2002) A multicentre, randomised phase III trial comparing protracted venous infusion (PVI) 5-fluorouracil (5-FU) with PVI 5-FU plus mitomycin C in patients with inoperable oesophago-gastric cancer. Ann Oncol 13:1568–1575
Bouché O, Raoul JL, Bonnetain F, Giovannini M, Etienne PL, Lledo G et al (2004) Randomized multicenter phase II trial of a biweekly regimen of fluorouracil and leucovorin (LV5FU2), LV5FU2 plus cisplatin, or LV5FU2 plus irinotecan in patients with previously untreated metastatic gastric cancer: a Federation Francophone de Cancerologie Digestive Group Study—FFCD 980. J Clin Oncol 22:4319–4328
Pozzo C, Barone C, Szanto J, Padi E, Peschel C, Bükki J et al (2004) Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol 15:1773–1781
Ajani JA, Fodor MB, Tjulandin SA, Moiseyenko VM, Chao Y, Cabral Filho S et al (2005) Phase II multi-institutional randomized trial of docetaxel plus cisplatin with or without fluorouracil in patients with untreated, advanced gastric, or gastroesophageal adenocarcinoma. J Clin Oncol 23:5660–5667
Moehler M, Eimermacher A, Siebler J, Hohler T, Wein A, Menges M et al (2005) Randomised phase II evaluation of irinotecan plus high-dose 5-fluorouracil and leucovorin (ILF) vs 5-fluorouracil, leucovorin, and etoposide (ELF) in untreated metastatic gastric cancer. Br J Cancer 92:2122–2128
Thuss-Patience PC, Kretzschmar A, Repp M, Kingreen D, Hennesser D, Micheel S et al (2005) Docetaxel and continuous-infusion fluorouracil versus epirubicin, cisplatin, and fluorouracil for advanced gastric adenocarcinoma: a randomized phase II study. J Clin Oncol 23:494–501
Chin K, Iishi H, Imamura H, Kobayashi O, Imamoto H, Esaki T et al (2007) Irinotecan plus S-1 (IRIS) versus S-1 alone as first line treatment for advanced gastric cancer: preliminary results of a randomized phase III study (GC0301/TOP-002). J Clin Oncol 25:15s, suppl; abstr 4525
Al-Batran SE, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R et al (2008) Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol 26:1435–1442
Dank M, Zaluski J, Barone C, Valvere V, Yalcin S, Peschel C et al (2008) Randomized phase III study comparing irinotecan combined with 5-fluorouracil and folinic acid to cisplatin combined with 5-fluorouracil in chemotherapy naive patients with advanced adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 19:1450–1457
Ikeda R, Yoshida K, Satou Y, Takahashi M, Une Y, Yamamoto W et al (2009) Randomized phase II/III study of docetaxel/S-1 (DS-1) versus CDDP/5FU (FUP) in advanced or recurrent gastric cancer: updated phase II results. J Clin Oncol 27:15s (suppl; abstr 4595)
Jeung H, Im C, Rha S, Ahn J, Shin S, Noh S et al (2008) A randomized phase II trial of docetaxel plus S-1 versus docetaxel plus cisplatin in advanced gastric cancer as a first-line treatment. J Clin Oncol 26 (suppl; abstr 4534)
Lee JL, Kang YK, Kang HJ, Lee KH, Zang DY, Ryoo BY et al (2008) A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer. Br J Cancer 99:584–590
Park SH, Nam E, Park J, Cho EK, Shin DB, Lee JH et al (2008) Randomized phase II study of irinotecan, leucovorin and 5-fluorouracil (ILF) versus cisplatin plus ILF (PILF) combination chemotherapy for advanced gastric cancer. Ann Oncol 19:729–733
Ridwelski K, Fahlke J, Kettner E, Schmidt C, Keilholz U, Quietzsch D et al (2008) Docetaxel-cisplatin (DC) versus 5-fluorouracil-leucovorin-cisplatin (FLC) as first-line treatment for locally advanced or metastatic gastric cancer: Preliminary results of a phase III study. J Clin Oncol 26 (suppl; abstr 4512)
Boku N, Yamamoto S, Fukuda H, Shirao K, Doi T, Sawaki A et al (2009) Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol 10:1063–1069
Lee KH, Hyun MS, Kim HK, Jin HM, Yang J, Song HS et al (2009) Randomized, multicenter, phase III trial of heptaplatin 1-hour infusion and 5-fluorouracil combination chemotherapy comparing with cisplatin and 5-fluorouracil combination chemotherapy in patients with advanced gastric cancer. Cancer Res Treat 41:12–18
Kang Y, Ohtsu A, Van Cutsem E, Rha SY, Sawaki A, Park S et al (2010) AVAGAST: A randomized, double-blind, placebo-controlled, phase III study of first-line capecitabine and cisplatin plus bevacizumab or placebo in patients with advanced gastric cancer (AGC). J Clin Oncol 28:18s (suppl; abstr LBA4007)
Kishimoto T, Imamura H, Uedou F, Fujitani K, Iijima S, Takiuchi H et al (2010) Randomized phase II trial of S-1 plus irinotecan versus S-1 plus paclitaxel as first-line treatment for advanced gastric cancer (OGSG0402): final report. J Clin Oncol 28:15s (suppl; abstr 4015)
Sawaki A, Yamaguchi K, Nabeya Y, Sakai Y, Osanai H, Denda T et al (2009) 5-FU/l-LV (RPMI) versus S1 as first-line therapy in patients with advanced gastric cancer: a randomized phase III non-inferiority trial. (ISO-5FU10 Study Group trial). Eur J Cancer (Supplements 7)363
Moehler M, Kanzler S, Geissler M, Raedle J, Ebert MP, Daum S et al (2010) A randomized multicenter phase II study comparing capecitabine with irinotecan or cisplatin in metastatic adenocarcinoma of the stomach or esophagogastric junction. Ann Oncol 21:71–77
Tebbutt NC, Cummins MM, Sourjina T, Strickland A, Van Hazel G, Ganju V et al (2010) Randomised, non-comparative phase II study of weekly docetaxel with cisplatin and 5-fluorouracil or with capecitabine in oesophagogastric cancer: the AGITG ATTAX trial. Br J Cancer 102:475–481
Fleming TR (2005) Surrogate endpoints and FDA’s accelerated approval process. Health Aff (Millwood) 24:67–68
Baker SG (2006) Surrogate endpoints: wishful thinking or reality? J Natl Cancer Inst 98:502–503
Ohtsu A (2007) Diverse eastern and Western approaches to the management of gastric cancer. Gastrointest Cancer Res 1(2 Suppl):S10–S15
Buyse M, Molenberghs G, Burzykowski T, Renard D, Geys H (2000) The validation of surrogate endpoints in meta-analyses of randomized experiments. Biostatistics 1:49–67
Acknowledgements
None.
Conflict of interest statement
None of the authors have financial or personal conflicts of interest to disclose.
Financial disclosure
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shitara, K., Ikeda, J., Yokota, T. et al. Progression-free survival and time to progression as surrogate markers of overall survival in patients with advanced gastric cancer: analysis of 36 randomized trials. Invest New Drugs 30, 1224–1231 (2012). https://doi.org/10.1007/s10637-011-9648-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-011-9648-y