Summary
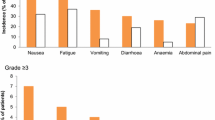
Background The California Cancer Consortium has performed a Phase II trial of infusional bryostatin, a protein kinase C inhibitor isolated from the marine invertebrate bryozoan, Bugula Neritina, a member of the phylum Ectoprocta, in combination with cisplatin, in patients (pts) with recurrent platinum-sensitive or resistant ovarian cancer (OC). Methods Pts received bryostatin 45 mcg/m2 as a 72 h continuous infusion followed by cisplatin 50 mg/m2. Cycles were repeated every 3 weeks. Dosages were chosen based on phase I data obtained by the CCC in a population of pts with mixed tumor types. Results Eight pts with recurrent or persistent epithelial OC received 23 cycles of treatment. All pts had received previous platinum-based chemotherapy; two pts had received one prior course, five had received two prior courses, and one had received three prior courses of chemotherapy. The median age was 64 (range 32–72), and Karnofsky performance status 90 (range 80–100). A median of 3 cycles of chemotherapy were delivered (range: 1–5). The median progression-free and overall survivals were 3 and 8.2 months respectively. Best responses included two partial responses (one in a platinum-resistant pt), three pts with stable disease, and three progressions. All pts experienced Grade 3 or 4 toxicities including severe myalgias/pain/fatigue/asthenia in six pts, and severe nausea/vomiting/constipation in two other pts. One pt experienced a seizure and liver function tests were elevated in one other. Conclusions A modest response rate is observed in pts with recurrent or persistent ovarian cancer treated with the combination of bryostatin and cisplatin. The toxicity profile, however, observed in this pt population (primarily severe myalgias), precludes tolerability and prevents this combination from further investigation at this dose and schedule. It is possible that platinum pre-exposure in OC patients exacerbates observed toxicity. Phase II dosages of investigational agents in OC pts that are determined by phase I trials in pts with other tumor types should be chosen cautiously.
Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ (2009) Cancer statistics, 2009. CA Cancer J Clin 59(4):225–249
Cannistra SA, Gershenson D, Recht A (2008) Ovarian cancer, fallopian tube carcinoma, and peritoneal carcinoma. In: DeVita VT Jr, Lawrence TS, Rosenberg S (eds) Cancer: principles and practice of oncology. Lippincott Williams & Wilkins, Philadelphia, pp 1568–1594
Alberts DS, Liu PY, Hannigan EV, O’Toole R, Williams SD, Young JA, Franklin EW, Clarke-Pearson DL, Malviya VK, DuBeshter B (1996) Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 335(26):1950–1955
Sabbatini P, Spriggs D (1998) Salvage therapy for ovarian cancer. Oncology 12(6):833–848
Stanwell C, Gescher A, Bradshaw TD, Pettit GR (1994) The role of protein kinase C isoenzymes in the growth inhibition caused by bryostatin 1 in human A549 lung and MCF-7 breast carcinoma cells. Int J Cancer 56:585–592
Hornung RL, Pearson JW, Beckwith M, Longo DL (1992) Preclinical evaluation of bryostatin as an anticancer agent against several murine tumor cell lines: in vitro versus in vivo activity. Cancer Res 52:101–107
Dale IL, Bradshaw TD, Gescher A, Pettit GR (1989) Comparison of effects of bryostatins 1 and 2 and 12-O- tetradecanoylphorbol-13-acetate on protein kinase C activity in A549 human lung carcinoma cells. Cancer Res 49:3242–3245
Jarvis WD, Povirk LF, Turner AJ, Traylor RS, Gewirtz DA, Pettit GR, Grant S (1994) Effects of bryostatin 1 and other pharmacological activators of protein kinase C on 1-[beta-D-arabinofuranosyl]cytosine-induced apoptosis in HL-60 human promyelocytic leukemia cells. Biochem Pharmacol 47:839–852
Asiedu C, Biggs J, Lilly M, Kraft AS (1995) Inhibition of leukemic cell growth by the protein kinase C activator bryostatin 1 correlates with the dephosphorylation of cyclin- dependent kinase 2. Cancer Res 55:3716–3720
Basu A, Lazo JS (1992) Sensitization of human cervical carcinoma cells to cis- diamminedichloroplatinum(II) by bryostatin 1. Cancer Res 52:3119–3124
Hofmann J, Doppler W, Jakob A, Maly K, Posch L, Uberall F, Grunicke HH (1988) Enhancement of the antiproliferative effect of cis- diamminedichloroplatinum(II) and nitrogen mustard by inhibitors of protein kinase C. Int J Cancer 42:382–388
Isonishi S, Andrews PA, Howell SB (1990) Increased sensitivity to cis-diamminedichloroplatinum(II) in human ovarian carcinoma cells in response to treatment with 12-O- tetradecanoylphorbol 13-acetate. J Biol Chem 265:3623–3627
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Green S, Weiss G (1992) Southwest Oncology Group standard response criteria, endpoint definitions and toxicity criteria. Invest New Drugs 10:239–253
Stanwell C (1994) The role of protein kinase C (PKC) modulation in the antiproliferative effects of bistratene A, bryostatin 1 and phorbol esters. Diss Abstr Int [C] 55:881
Dale IL, Gescher A (1989) Effects of activators of protein kinase C, including bryostatins 1 and 2, on the growth of A549 human lung carcinoma cells. Int J Cancer 43:158–163
Schuchter LM, Esa AH, May S, Laulis MK, Pettit GR, Hess AD (1991) Successful treatment of murine melanoma with bryostatin 1. Cancer Res 51:682–687
Varterasian ML, Mohammad RM, Eilender DS, Hulburd K, Rodriguez DH, Pemberton PA, Pluda JM, Dan MD, Pettit GR, Chen BD, Al-Katib AM (1998) Phase I study of bryostatin 1 in patients with relapsed non- Hodgkin’s lymphoma and chronic lymphocytic leukemia. J Clin Oncol 16:56–62
Basu A, Teicher BA, Lazo JS (1990) Involvement of protein kinase C in phorbol ester-induced sensitization of HeLa cells to cis-diamminedichloroplatinum(II). J Biol Chem 265:8451–8457
Martiny-Baron G, Fabbro D (2007) Classical PKC isoforms in cancer. Pharmacol Res 55:477–486
Serova M, Ghoul A, Benhadji KA, Cvitkovic E, Faivre S, Calvo F, Lokiec F, Raymond E (2006) Preclinical and clinical development of novel agents that target the protein kinase C Family. Sem Oncol 33:466–478
Hanauske A-R, Sundell K, Lahn M (2004) The role of protein kinases C-alpha (PKC-α) in cancer and its modulation by the novel PKC-α-specific inhibitor aprinocarsen. Curr Pharm Des 10(16):1923–1936
Pavlick AC, Wu J, Roberts J, Rosenthal MA, Hamilton A, Wadler S, Farrell K, Carr M, Fry D, Murgo AJ, Oratz R, Hochster H, Liebes L, Muggia F (2009) Phase I study of bryostatin 1, a protein kinase C modulator, preceding cisplatin in patients with refractory non-hematologic tumors. Cancer Chemother Pharmacol 64:803–810
Hickman PF, Kemp GJ, Thompson CH, Salisbury AJ, Wade K, Harris AL, Radda GK (1995) Bryostatin 1, a novel antineoplastic agent and protein kinase C activator, induces human myalgia and muscle metabolic defects: a 31P magnetic resonance spectroscopic study. Br J Cancer 72:998–1003
Thompson CH, Macaulay VM, O’Byrne KJ, Kemp GJ, Wilner SM, Talbot DC, Harris AL, Radda GK (1996) Modulation of bryostatin 1 muscle toxicity by nifedipine: effects on muscle metabolism and oxygen supply. Br J Cancer 73:1161–1165
Lenz HJ, Raghavan D, Doroshow J, Gandara DR (1999) Phase I study of bryostatin-1 in combination with cisplatin in treating patients with metastatic or unresectable solid tumors including non small-cell lung cancer. Clin Lung Cancer 1(2):151–152
Author information
Authors and Affiliations
Corresponding author
Additional information
Reported in abstract form in: Proc. Amer. Soc. Clin. Oncol. 23:5140, 2005.
Supported by NCI Cancer Center Core Grant CA 33572, CA 63265, and CA 62505 from the NCI and NCI Contract N01-CM-17101.
Rights and permissions
About this article
Cite this article
Morgan, R.J., Leong, L., Chow, W. et al. Phase II trial of bryostatin-1 in combination with cisplatin in patients with recurrent or persistent epithelial ovarian cancer: a California cancer consortium study. Invest New Drugs 30, 723–728 (2012). https://doi.org/10.1007/s10637-010-9557-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9557-5