Summary
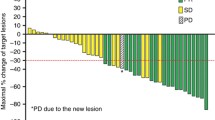
Introduction Vorinostat is an inhibitor of histone deacetylase 6, which acetylates tubulin and stabilizes microtubules. Since taxanes also stabilize microtubules, we hypothesized that the administration of vorinostat followed by docetaxel should result in synergistic cytotoxicity. We conducted a phase I trial to determine the dose level of vorinostat plus docetaxel that would result in dose-limiting toxicity (DLT) in ≤30% of patients. Methods Eligible patients had castration-resistant prostate cancer (CRPC) or relapsed urothelial or non-small-cell lung cancer (NSCLC) after ≥1 prior chemotherapy regimen not containing docetaxel, performance status of 0–2, and adequate organ function. Vorinostat was given orally for 14 days beginning on day 1 of a 21-day cycle, with docetaxel given intravenously over 1 h on day 4. The time-to-event continuous reassessment method (TITE-CRM) guided dose escalation. Dose levels (DL) -1, 0, 1 and 2 corresponded to vorinostat 100, 100, 200 and 200 mg plus docetaxel 50, 60, 60, and 75 mg/m2, respectively. Pharmacokinetic studies were performed on days 1 and 4 of cycle 1. Results Twelve patients were enrolled: median age 65 years (range 49–74); 9 male, 3 female; 4 CRPC, 5 urothelial, 3 NSCLC. The median number of cycles administered was 2. Two patients were treated at DL -1, 4 at DL 0, 5 at DL 1 and 1 at DL 2. Five DLTs occurred in 5 patients: neutropenic fever/sepsis (2), anaphylactic reaction (1), myocardial infarction (1) and gastrointestinal bleed (1). Other toxicities included grade 3/4 neutropenia (4), peripheral neuropathy (1), and gastrointestinal bleed (n = 1). The estimated probability of DLT for DL -1 was 0.32 (90% posterior probability interval [PI], 0.11 to 0.53) for DL 0, 0.38 (90% PI, 0.16 to 0.58) and for DL 1, 0.43 (90% PI, 0.23 to 0.64). The trial was stopped due to excessive toxicity. No responses were noted. Conclusions The combination of vorinostat and docetaxel was poorly tolerated with excessive DLTs that required early study termination. No responses were identified. Vorinostat and docetaxel pharmacokinetics were comparable to previous reports in the literature, without obvious drug-drug interactions.

Similar content being viewed by others
References
Workman JL, Kingston RE (1998) Alteration of nucleosome structure as a mechanism of transcriptional regulation. Annu Rev Biochem 67:545–579
Arts J, de Schepper S, Van Emelen K (2003) Histone deacetylase inhibitors: from chromatin remodeling to experimental cancer therapeutics. Curr Med Chem 10:2343–2350
Hess-Stumpp H (2005) Histone deacetylase inhibitors and cancer: from cell biology to the clinic. Eur J Cell Biol 84:109–121
Jones PA, Baylin SB (2002) The fundamental role of epigenetic events in cancer. Nat Rev Genet 3:415–428
Xu W, Ngo L, Perez G et al (2006) Intrinsic apoptotic and thioredoxin pathways in human prostate cancer cell response to histone deacetylase inhibitors. Proc Natl Acad Sci USA 103:15540–15545
Dokmanovic M, Clarke C, Marks PA (2007) Histone deacetylase inhibitors: overview and perspectives. Mol Cancer Res 5:981–989
Finnin MS, Donigian JR, Cohen A et al (1999) Structures of a histone deacetylase homologue bound to the TSA and vorinostat inhibitors. Nature 401:188–193
Marks P, Rifkind RA, Richon VM et al (2001) Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer 1:194–202
Kelly WK, O’Connor OA, Marks PA et al (2002) Histone deacetylase inhibitors: from target to clinical trials. Expert Opin Investig Drugs 11:1695–1713
Cohen LA, Marks PA, Rifkind RA et al (2002) Suberoylanilide hydroxamic acid (SAHA), a histone deacetylase inhibitor, suppresses the growth of carcinogen-induced mammary tumors. Anticancer Res 22:1497–1504
Bali P, Pranpat M, Swaby R et al (2005) Activity of suberoylanilide hydroxamic acid against human breast cancer cells with amplification of Her-2. Clin Cancer Res 11:6382–6389
Mann BS, Johnson JR, He K et al (2007) Vorinostat for treatment of cutaneous manifestations of advanced primary cutaneous T-cell lymphoma. Clin Cancer Res 13:2318–2322
Peart MJ, Tainton KM, Ruefli AA et al (2003) Novel mechanisms of apoptosis induced by histone deacetylase inhibitors. Cancer Res 63:4460–4471
Butler LM, Agus DB, Scher HI et al (2000) Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, suppresses the growth of prostate cancer cells in vitro and in vivo. Cancer Res 60:5165–5170
Seo SK, Jin HO, Lee HC et al (2008) Combined effects of sulindac and suberoylanilide hydroxamic acid on apoptosis induction in human lung cancer cells. Mol Pharmacol 73:1005–1012
Krug LM, Curley T, Schwartz L et al (2006) Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer 7:257–261
Zhang C, Richon V, Ni X et al (2005) Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol 125:1045–1052
Palazzo A, Ackerman B, Gundersen G (2002) Cell biology: tubulin acetylation and cell motility. Nature 421:230
Hubbert C, Guardiola A, Shao R et al (2002) HDAC6 is a microtubule-associated deacetylase. Nature 417:455–458
Zhang Y, Li N, Caron C et al (2003) HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. Embo J 22:1168–1179
Ringel I, Horwitz SB (1991) Studies with RP 56976 (Taxotere): A semisynthetic analogue of taxol. J Natl Cancer Inst 83:288–291
Rao S, Krauss NE, Heerding JM et al (1994) 3′-(p-azidobenzamido)taxol photolabels the N-terminal 31 amino acids of beta-tubulin. J Biol Chem 269:3132–3134
Rao S, Orr GA, Chaudhary AG et al (1995) Characterization of the Taxol Binding Site on the Microtubule: 2-(m-AZIDOBENZOYL)TAXOL PHOTOLABELS A PEPTIDE (AMINO ACIDS 217-231) of β-TUBULIN. J Biol Chem 270:20235–20238
Rao S, He L, Chakravarty S et al (1999) Characterization of the Taxol Binding Site on the Microtubule: IDENTIFICATION OF Arg282 IN β-TUBULIN AS THE SITE OF PHOTOINCORPORATION OF A 7-BENZOPHENONE ANALOGUE OF TAXOL. J Biol Chem 274:37990–37994
Nogales E, Wolf SG, Downing KH et al (1998) Structure of the |[alpha]||[beta]| tubulin dimer by electron crystallography. Nature 391:199–203
Zhou J, Giannakakou P (2005) Targeting microtubules for cancer chemotherapy. Curr Med Chem–Anti-Cancer Agents 5:65–71
Blagosklonny MV, Robey R, Sackett DL et al (2002) Histone deacetylase inhibitors all induce p21 but differentially cause tubulin acetylation, mitotic arrest, and cytotoxicity. Mol Cancer Ther 1:937–941
Baker SD, Sparreboom A, Verweij J (2006) Clinical pharmacokinetics of docetaxel recent developments. Clin Pharmacokinet 45:235–252
Rubin EH, Agrawal NG, Friedman EJ et al (2006) A study to determine the effects of food and multiple dosing on the pharmacokinetics of vorinostat given orally to patients with advanced cancer. Clin Cancer Res 12:7039–7045
Cockroft DW, Gauld MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Goodman SN, Zahurak ML, Piantadosi S et al (1995) Some practical improvements in the continual reassessment method for phase I studies. Stat Med 14:1149–1161
O’Quigley J, Shen LZ (1996) Continual reassessment method: a likelihood approach. Biometrics 52:673–684
Cheung YK, Chappell R (2000) Sequential designs for phase I clinical trials with late-onset toxicities. Biometrics 56:1177–1182
Parise RA, Holleran JL, Beumer JH et al (2006) A liquid chromatography-electrospray ionization tandem mass spectrometric assay for quantitation of the histone deacetylase inhibitor, vorinostat (suberoylanilide hydroxamicacid, SAHA), an its metabolites in human serum. J Chromatogr B Analyt Technol Biomed Life Sci 840:108–115
Parise RA, Ramanathan RK, Zamboni WC et al (2003) Sensitive liquid chromatography-mass spectrometry assay fro quantitation of docetaxel and paclitaxel in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 783:231–236
Yeh KC, Kwan KC (1978) A comparison of numerical integrating algorithms by trapezoidal, lagrange, and spline approximation. J Pharmacokinet Biopharm 6:79–98
Rocci ML Jr, Jusko WJ (1983) LAGRAN program for area and moments in pharmacokinetic analysis. Comput Programs Biomed 16:203–216
Florian JA, Zamboni WC, Eiseman JL, et al. A physiologically-based pharmacokinetic model for docetaxel distribution in SCID mice bearing SKOV-3 human ovarian cancer xenografts. J Pharmacokinet Pharmacodynam (in press)
D’Argenio DZ, Schumitzky A (1997) Adapt II user’s guide: pharmacokinetic/Pharmacodynamic systems analysis software. Los Angeles (CA): Biomedical Simulations Resource
Kloft C, Wallin J, Henningsson A et al (2006) Population pharmacokinetic-pharmacodynamic model for neutropenia with patient subgroup identification: comparison across anticancer drugs. Clin Cancer Res 12:5481–5490
Bruno R, Hille D, Riva A et al (1998) Population pharmacokinetics/pharmacodynamics of docetaxel in phase II studies in patients with cancer. J Clin Oncol 16:187–196
Jones PA, Baylin SB (2007) The epigenomics of cancer. Cell 128:683–692
Yoo CB, Jones PA (2006) Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov 5:37–50
Owonikoko TK, Ramalingam SS, Kanterewicz B et al (2010) Vorinostat increases carboplatin and paclitaxel activity in non-small-cell lung cancer cells. Int J Cancer 126:743–755
Kelly WK, O’Connor OA, Krug LM et al (2005) Phase I study of an oral histone deacetylase inhibitor, Suberoylanilide hydroxamic acid, in patients with advanced cancer. J Clin Oncol 23:3923–3931
Cheung EM, Quinn DI, Tsao-Wei DD, et al (2008) Phase II study of vorinostat (Suberoylanilide Hydroxamic Acid, SAHA) in patients with advanced transitional cell urothelial cancer (TCC) after platinum-based therapy—California Cancer Consortium/University of Pittsburgh NCI/CTEP-sponsored trial. J Clin Oncol 26 (May 20 suppl) abstr 16058
Bradley DA, Rathkopf D, Dunn R et al (2009) Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862). Cancer 115:5541–5549
Shepherd FA, Dancey J, Ramlau R et al (2000) Prospective randomized trial of docetaxel versus best supportive care in patients with non-small-cell lung cancer previously treated with platinum-based chemotherapy. J Clin Oncol 18:2095–2103
Fossella FV, DeVore R, Kerr RN et al (2000) Randomized phase III trial of docetaxel versus vinorelbine or ifosfamide in patients with advanced non-small-cell lung cancer previously treated with platinum-containing chemotherapy regimens. J Clin Oncol 18:2354–2362
Ramalingam SS, Parise RA, Ramananthan RK et al (2007) Phase I and pharmacokinetic study of vorinostat, a histone deacetylase inhibitor, in combination with carboplatin and paclitaxel for advanced solid malignancies. Clin Cancer Res 13:3605–3610
Ramalingam SS, Maitland ML, Frankel P et al (2010) Carboplatin and paclitaxel in combination with either vorinostat or placebo for first-line therapy of advanced non-small-cell lung cancer. J Clin Oncol 28:56–62
Traynor AM, Dubey S, Eickhoff JC et al (2009) Vorinostat (NSC# 701852) in patients with relapsed non-small cell lung cancer: a Wisconsin Oncology Network Phase II Study. J Thorac Oncol 4:522–526
Petrylak DP, Tangen CM, Hussain MH et al (2004) Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 351:1513–1520
Cooper AL, Greenberg VL, Lancaster PS et al (2007) In vitro and in vivo histone deacetylase inhibitor therapy with suberoylanilide hydroxamic acid (SAHA) and paclitaxel in ovarian cancer. Gynecol Oncol 104:596–601
Miyanaga A, Gemma A, Noro R et al (2008) Anti-tumor activity of histone deacetylase inhibitors in non-small cell lung cancer cells: development of a molecular predictive model. Mol Cancer Ther 7:1923–1930
Author information
Authors and Affiliations
Corresponding author
Additional information
Supported in part by a grant from Merck & Co., Inc.
Rights and permissions
About this article
Cite this article
Schneider, B.J., Kalemkerian, G.P., Bradley, D. et al. Phase I study of vorinostat (suberoylanilide hydroxamic acid, NSC 701852) in combination with docetaxel in patients with advanced and relapsed solid malignancies. Invest New Drugs 30, 249–257 (2012). https://doi.org/10.1007/s10637-010-9503-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9503-6