Summary
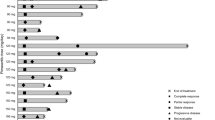
Purpose A Phase II study to screen for anti-melanoma activity of the heat shock protein 90 (HSP90) inhibitor, 17-AAG (17-allylamino-17-demethoxygeldanamycin) was performed. The primary endpoint was the rate of disease stabilisation in patients with progressive, metastatic melanoma treated with 17-AAG. Secondary endpoints were to determine: the toxicity of 17-AAG, the duration of response(s), median survival and further study the pharmacokinetics and pharmacodynamics of 17-AAG. Patients and Methods Patients with metastatic melanoma (progressive disease documented ≤6 months of entering study) were treated with weekly, intravenous 17-AAG. A Simon one sample two stage minimax design was used. A stable disease rate of ≥25% at 6 months was considered compatible with 17-AAG having activity. Results Fourteen patients (8 male: 6 female) were entered, eleven received 17-AAG (performance status 0 or 1). Median age was 60 (range 29–81) years. The majority (93%) received prior chemotherapy and had stage M1c disease (71%). Toxicity was rarely ≥ Grade 2 in severity and commonly included fatigue, headache and gastrointestinal disturbances. One of eleven patients treated with 17-AAG had stable disease for 6 months and median survival for all patients was 173 days. The study was closed prematurely prior to completion of the first stage of recruitment and limited planned pharmacokinetic and pharmacodynamic analyses. Conclusion Some evidence of 17-AAG activity was observed although early study termination meant study endpoints were not reached. Stable disease rates can be incorporated into trials screening for anti-melanoma activity and further study of HSP90 inhibitors in melanoma should be considered.



Similar content being viewed by others
References
Eigentler TK, Caroli UM, Radny P et al (2003) Palliative therapy of disseminated malignant melanoma: a systematic review of 41 randomised clinical trials. Lancet Oncol 4:748–759
Pearl LH, Prodromou C, Workman P (2008) The Hsp90 molecular chaperone: an open and shut case for treatment. Biochem J 410:439–453
Neckers L, Neckers K (2005) Heat-shock protein 90 inhibitors as novel cancer chemotherapeutics—an update. Expert Opin Emerg Drugs 10:137–149
Workman P, Burrows F, Neckers L et al (2007) Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci 1113:202–216
Powers MV, Workman P (2007) Inhibitors of the heat shock response: biology and pharmacology. FEBS Lett 581:3758–3769
Banerji U, Walton M, Raynaud F, Grimshaw R, Kelland L, Valenti M, Judson I, Workman P (2005) Pharmacokinetic-pharmacodynamic relationships for the heat shock protein 90 molecular chaperone inhibitor 17-allylamino, 17-demethoxygeldanamycin in human ovarian cancer xenograft models. Clin Cancer Res 11(19 Pt 1):7023–7032
Banerji U, Sain N, Sharp SY, Valenti M, Asad Y, Ruddle R, Raynaud F, Walton M, Eccles SA, Judson I, Jackman AL, Workman P (2008) An in vitro and in vivo study of the combination of the heat shock protein inhibitor 17-allylamino-17-demethoxygeldanamycin and carboplatin in human ovarian cancer models. Cancer Chemother Pharmacol 62(5):769–778
Solit DB, Zheng FF, Drobnjak M et al (2002) 17-Allylamino-17-demethoxygeldanamycin induces the degradation of androgen receptor and HER-2/neu and inhibits the growth of prostate cancer xenografts. Clin Cancer Res 8:986–993
Sharp SY, Boxall K, Rowlands M, Prodromou C, Roe SM, Maloney A, Powers M, Clarke PA, Box G, Sanderson S, Patterson L, Matthews TP, Cheung KM, Ball K, Hayes A, Raynaud F, Marais R, Pearl L, Eccles S, Aherne W, McDonald E, Workman P (2007) In vitro biological characterization of a novel, synthetic diaryl pyrazole resorcinol class of heat shock protein 90 inhibitors. Cancer Res 67(5):2206–2216
Sharp SY, Prodromou C, Boxall K et al (2007) Inhibition of the heat shock protein 90 molecular chaperone in vitro and in vivo by novel, synthetic, potent resorcinylic pyrazole/isoxazole amide analogues. Mol Cancer Ther 6:1198–1211
Banerji U, O’Donnell A, Scurr M et al (2005) Phase I pharmacokinetic and pharmacodynamic study of 17-allylamino, 17-demethoxygeldanamycin in patients with advanced malignancies. J Clin Oncol 23:4152–4161
Solit DB, Ivy SP, Kopil C et al (2007) Phase I trial of 17-allylamino-17-demethoxygeldanamycin in patients with advanced cancer. Clin Cancer Res 13:1775–1782
Workman P (2003) Auditing the pharmacological accounts for Hsp90 molecular chaperone inhibitors: unfolding the relationship between pharmacokinetics and pharmacodynamics. Mol Cancer Ther 2:131–138
Moreno-Farre J, Asad Y, Pacey S et al (2006) Development and validation of a liquid chromatography/tandem mass spectrometry method for the determination of the novel anticancer agent 17-DMAG in human plasma. Rapid Commun Mass Spectrom 20:2845–2850
Pacey S, Walton M, Eisen T et al (2006) Validation and use of western blotting to measure pharmacodynamic endpoints in Cancer Research UK clinical trials of Heat Shock Protein (HSP90) inhibitors. Abstract A161. Presented at the NCRI meeting, Birmingham, UK
Smith V, Sausville EA, Camalier RF et al (2005) Comparison of 17-dimethylaminoethylamino-17-demethoxy-geldanamycin (17DMAG) and 17-allylamino-17-demethoxygeldanamycin (17AAG) in vitro: effects on Hsp90 and client proteins in melanoma models. Cancer Chemother Pharmacol 56:126–137
Burger AM, Fiebig HH, Stinson SF et al (2004) 17-(Allylamino)-17-demethoxygeldanamycin activity in human melanoma models. Anticancer Drugs 15:377–387
Flaherty K, Puzanov I, Sosman J et al (2009) Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer. J Clin Oncol 27:9000
da Rocha Dias S, Friedlos F, Light Y, Springer C, Workman P, Marais R (2005) Activated B-RAF is an Hsp90 client protein that is targeted by the anticancer drug 17-allylamino-17-demethoxygeldanamycin. Cancer Res 65(23):10686–10691
Modi S, Stopeck AT, Gordon MS et al (2007) Combination of trastuzumab and tanespimycin (17-AAG, KOS-953) is safe and active in trastuzumab-refractory HER-2 overexpressing breast cancer: a phase I dose-escalation study. J Clin Oncol 25:5410–5417
Davies H, Bignell GR, Cox C (2002) et al Mutations of the BRAF gene in human cancer. Nature 417:949–954
Banerji U, Affolter A, Judson I, Marais R, Workman P (2008) BRAF and NRAS mutations in melanoma: potential relationships to clinical response to HSP90 inhibitors. Mol Cancer Ther 7(4):737–739
Nowakowski GS, McCollum AK, Ames MM et al (2006) A phase I trial of twice-weekly 17-allylamino-demethoxy-geldanamycin in patients with advanced cancer. Clin Cancer Res 12:6087–6093
Gelmon KA, Eisenhauer EA, Harris AL et al (1999) Anticancer agents targeting signaling molecules and cancer cell environment: challenges for drug development? J Natl Cancer Inst 91:1281–1287
Michaelis LC, Ratain MJ (2007) Phase II trials published in 2002: a cross-specialty comparison showing significant design differences between oncology trials and other medical specialties. Clin Cancer Res 13:2400–2405
Middleton MR, Grob JJ, Aaronson N et al (2000) Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. J Clin Oncol 18:158–166
Richards JM, Gale D, Mehta N et al (1999) Combination of chemotherapy with interleukin-2 and interferon alfa for the treatment of metastatic melanoma. J Clin Oncol 17:651–657
Balch CM, Soong SJ, Gershenwald JE et al (2001) Prognostic factors analysis of 17, 600 melanoma patients: validation of the American Joint Committee on Cancer melanoma staging system. J Clin Oncol 19:3622–3634
Manola J, Atkins M, Ibrahim J et al (2000) Prognostic factors in metastatic melanoma: a pooled analysis of Eastern Cooperative Oncology Group trials. J Clin Oncol 18:3782–3793
Gogas HJ, Kirkwood JM, Sondak VK (2007) Chemotherapy for metastatic melanoma: time for a change? Cancer 109:455–464
Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10:1–10
Therasse P (2002) Evaluation of response: new and standard criteria. Ann Oncol 13(Suppl 4):127–129
Egorin MJ, Zuhowski EG, Rosen DM et al (2001) Plasma pharmacokinetics and tissue distribution of 17-(allylamino)-17-demethoxygeldanamycin (NSC 330507) in CD2F1 mice1. Cancer Chemother Pharmacol 47:291–302
Kamal A, Thao L, Sensintaffar J et al (2003) et al A high-affinity conformation of Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature 425:407–410
Chung YL, Troy H, Banerji U et al (2003) Magnetic Resonence Spectroscopic Pharmacodynamic Markers of the Heat Shock Protien Inhibitor 17-Allyamino, 17-Demethoxygeldanamycin (17-AAG) in Human Colon Cancer Models. J Natl Cancer Inst 95:1624–1633
Smith-Jones PM, Solit D, Afroze F et al (2006) Early tumor response to Hsp90 therapy using HER2 PET: comparison with 18F-FDG PET. J Nucl Med 47:793–796
Taldone T, Gozman A, Maharaj R et al (2008) Targeting Hsp90: small-molecule inhibitors and their clinical development. Curr Opin Pharmacol 8:370–374
Kefford R, Millwars M, Hersey P et al (2007) Phase 2 Trial of Tanespimycin (KOS-953), a Heat Shock Protein-90 (Hap90) Inhibitor in Patients with Metastatic Melanoma. J Clin Oncol 25:8558
Pacey S, Wilson R, Walton M et al (2007) A Phase I Trial Of The Heat Shock Protein 90 (HSP90) Inhibitor 17-Dimethylaminoethylamino-17-Demethoxygeldanamycin (17-DMAG, Alvespimycin) Administered Weekly. J Clin Oncol 25
Flaherty KT, Gore L, Avadhani A et al (2008) First use of an oral Hsp90 inhibitor in patients (Pts) with solid tumors: Alvespimycin (A) administered QOD or QD. J Clin Oncol 26:2502
Solit DB, Osman I, Polsky D et al (2008) Phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with metastatic melanoma. Clin Cancer Res 14:8302–8307
Korn EL, Liu PY, Lee SJ et al (2008) Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 26:527–534
Elfiky A, Saif MW, Beeram M et al (2008) BIIB021, an oral, synthetic non-ansamycin Hsp90 inhibitor: Phase I experience. J Clin Oncol 26:2503
Sessa C, Sharma SK, Britten CD et al (2009) A phase I dose escalation study of AUY922, a novel HSP90 inhibitor, in patients with advanced solid malignancies. J Clin Oncol 27:3532
Bryson JC, Infante JR, Ramanathan RK et al (2008) A phase 1 dose-escalation study for the safety and pharmacokinetics (PK) of the oral Hsp90 inhibitor SNX-4522. J Clin Oncol 26:14613
Eccles SA, Massey A, Raynaud FI, Sharp SY, Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall F, Aherne W, Rowlands M, Hayes A, Martins V, Urban F, Boxall K, Prodromou C, Pearl L, James K, Matthews TP, Cheung KM, Kalusa A, Jones K, McDonald E, Barril X, Brough PA, Cansfield JE, Dymock B, Drysdale MJ, Finch H, Howes R, Hubbard RE, Surgenor A, Webb P, Wood M, Wright L, Workman P (2008) NVP-AUY922: a novel heat shock protein 90 inhibitor active against xenograft tumor growth, angiogenesis, and metastasis. Cancer Res 68(8):2850–2860
Acknowledgements
This trial was performed under the sponsorship and management of Cancer Research UK’s Drug Development Office. The authors would like to acknowledge the contribution of the staff of; the Melanoma and the Drug Development Units (Royal Marsden Hospital, UK), the Melanoma unit (The Royal Free Hospital, London, UK) as well as the staff of the pharmacy units at both hospitals and the Drug Development Office of Cancer Research UK (London, UK). In particular, we acknowledge Sarah Stapleton, Maggie James, Jane Vincent and Lindsey Gumbrell. The protocol concept was developed at the 2002 NCI/EORTC/AACR Protocol Development Workshop (Flims, Switzerland) by Dr Udai Banerji. This work was supported by Cancer Research UK programme grant numbers C309/A8274, C212/A5720, C212/A7324 C212/A7324 and C212/A11342. Paul Workman is a Cancer Research UK Life Fellow and Simon Pacey was the grateful recipient of a Cancer Research UK New Agents Committee Clinical Research Fellowship. With respect to conflict of interest, the following are noted in the paper: Professor Paul Workman and his team received research funding on the development of HSP90 inhibitors from Vernalis Ltd and intellectual property from this programme was licensed to Vernalis Ltd and Novartis. Pacey, Judson, Banerji, Raynaud, Asad, Walton and Workman are/were employees of The Institute of Cancer Research which has a commercial interest in HSP90 inhibitors under development by Novartis Ltd. 17-AAG was supplied by the NCI and Kosan Biosciences Ltd. Most of all, we thank our patients, their families and friends for their support and participation in our early clinical trials.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pacey, S., Gore, M., Chao, D. et al. A Phase II trial of 17-allylamino, 17-demethoxygeldanamycin (17-AAG, tanespimycin) in patients with metastatic melanoma. Invest New Drugs 30, 341–349 (2012). https://doi.org/10.1007/s10637-010-9493-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-010-9493-4