Summary
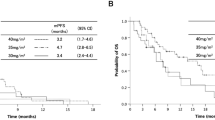
Amrubicin is a totally synthetic anthracycline anticancer drug and a potent topoisomerase II inhibitor. Recently, amrubicin was approved in Japan for the treatment of small- and non-small-cell lung cancers (SCLC and NSCLC). Here, we review the efficacy and toxicities of amrubicin monotherapy and amrubicin in combination with cisplatin for extensive-disease SCLC (ED-SCLC), and of amrubicin monotherapy for advanced NSCLC, as observed in the clinical trials. Recommended dosage for previously untreated advanced NCSLC was 45 mg/m2/day by intravenous administration for 3 days. Dose-limiting toxicities were leucopenia, thrombocytopenia, and gastrointestinal disturbance. Response rate was 27.9% for advanced NSCLC, and 75.8% for ED-SCLC with a median survival time (MST) of 11.7 months. Recommended dosage of amrubicin was 40 mg/m2/day in combination with cisplatin at 60 mg/m2/day, with MST of 13.6 months and 1-year survival rate of 56.1%. In sensitive or refractory relapsed SCLC, response rate was 52 and 50%, progression-free survival was 4.2 and 2.6 months, overall survival was 11.6 and 10.3 months, and 1-year survival rate was 46 and 40%, respectively. These results are promising for the treatment of both NSCLC and SCLC. Further clinical trials will clarify the status of amrubicin in the treatment of lung cancer.

Similar content being viewed by others
References
Yamaoka T, Hanada M, Ichii S et al (1998) Cytotoxicity of amrubicin, a novel 9-aminoanthracycline, and its metabolite amrubicinol on human tumor cells. Jpn J Cancer Res 89:1067–1073
Noguchi T, Ichii S, Yamaoka T et al (1998) In vivo efficacy and tumor-selective metabolism of amrubicin to its active metabolite. Jpn J Cancer Res 89:1055–1060
Suzuki T, Minamide S, Iwasaki T et al (1997) Cardiotoxicity of a new anthracycline derivative (SM5887) following intravenous administration to rabbits: comparative study with doxorubicin. Invest New Drugs 15:219–225
Morisada S, Yanagi Y, Noguchi T et al (1989) Antitumor activities of a novel 9-aminoanthracycline (SM5887) against mouse experimental tumors and human tumor xenografts. Jpn J Cancer Res 80:69–76
Noguchi T, Ichii S, Morisada S et al (1999) Evaluation of amrubicin with a 5 day administration schedule in a mouse model. Gan To Kagaku Ryoho 26:1305–1312
Hanada M, Mizuno S, Fukushima A et al (1998) A new antitumor agent amrubicin induces cell growth inhibition by stabilizing topoisomerase II-DNA complex. Jpn J Cancer Res 89:1229–1238
Inoue K, Ogawa M, Horikoshi N et al (1988) Phase I study of SM-5887, a new anthracycline derivative. Gan To Kagaku Ryoho 15:1771–1776
Inoue K, Matsumura A, Horikoshi N et al (1992) Phase I and pharmacokinetic study of SM-5887 by 5-day schedule. Gan To Kagaku Ryoho 19:477–482
Feld R, Wierzbicki R, Walde PLD et al (1992) Phase I–II study of high-dose epirubicin in advanced non-small-cell lung cancer. J Clin Oncol 10:297–303
Sugiura T, Ariyoshi Y, Negoro S et al (2005) Phase I/II study of amrubicin, a novel 9-aminoanthracycline, in patients with advanced non-small-cell lung cancer. Invest New Drugs 23:331–337
Sawa T, Yana T, Takada M et al (2006) Multicenter phase II study of amrubicin, 9-amino-anthracycline, in patients with advanced non-small-cell lung cancer (Study 1): West Japan Thoracic Oncology Group (WJTOG) trial. Invest New Drugs 24(2):151–158
Yana T, Negoro S, Takada M et al (2006) Phase II study of amrubicin in previously untreated patients with extensive-disease small cell lung cancer: West Japan Thoracic Oncology Group (WJTOG) study. Invest New Drugs 25:253–258
Ohe Y, Negoro S, Matsui K et al (2005) Phase I–II study of amrubicin and cisplatin in previously untreated patients with extensive-stage small-cell lung cancer. Ann Oncol 16:430–436
Noda K, Nishiwaki Y, Kawahara M et al (2005) Irinotecan plus cisplatin compared with etoposide plus cisplatin for extensive small-cell lung cancer. N Engl J Med 346:85–91
Onoda S, Masuda N, Seto T et al (2006) Phase II trial of amrubicin for treatment of refractory or relapsed small-cell lung cancer: Thoracic Oncology Research Group Study 0301. J Clin Oncol 24(34):5448–5453
Glisson BS (2003) Recurrent small cell lung cancer: update. Semin Oncol 30(1):72–78
von Pawel J, Schiller JH, Shepherd FA et al (1999) Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol 17(2):658–667
Holmes FA, Madden T, Newman RA et al (1996) Sequence-dependent alteration of doxorubicin pharmacokinetics by paclitaxel in a phase I study of paclitaxel and doxorubicin in patients with metastatic breast cancer. J Clin Oncol 14:2713–2721
Yanaihara T, Yokoba M, Masuda N et al (2007) Phase I and pharmacologic study of irinotecan and amrubicin in advanced non-small cell lung cancer. Cancer Chemother Pharmacol 59(4):419–427
Okamoto I, Hamada A, Matsunaga Y et al (2005) Phase I and pharmacokinetic study of amrubicin, a synthetic 9-aminoanthracycline, in patients with refractory or relapsed lung cancer. Cancer Chemother Pharmacol 19:1–7, July
Matsunaga Y, Hamada A, Okamoto I et al (2006) Pharmacokinetics of amrubicin and its active metabolite amrubicinol in lung cancer patients. Ther Drug Monit 28(1):76–82
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurata, T., Okamoto, I., Tamura, K. et al. Amrubicin for non-small-cell lung cancer and small-cell lung cancer. Invest New Drugs 25, 499–504 (2007). https://doi.org/10.1007/s10637-007-9069-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-007-9069-0