Summary
Background: LY293111 is an oral agent known to be a leukotriene B4 (LTB4) receptor antagonist and a 5-lipoxygenase inhibitor resulting in selective inhibition of the lipoxygenase pathway. Lipoxygenases metabolize arachidonic acid and have been involved in cancer cell proliferation and survival. In addition, LY293111 has been found to be a peroxisome proliferator activated receptor-gamma (PPAR-γ) agonist. Antineoplastic activity of LY293111 has been identified in preclinical models both alone and in combination with chemotherapy agents including irinotecan. The NCIC Clinical Trials Group studied LY293111 in combination with irinotecan to determine the recommended dose of the combination and to describe its tolerability and pharmacokinetic interaction. In addition the anti-tumour activity of LY293111 in combination with irinotecan was documented.
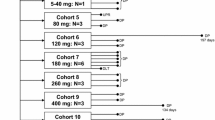
Patients and methods: Twenty-eight patients with advanced solid tumours were treated on seven dose levels with the combination of irinotecan and LY293111. Irinotecan was administered intravenously every 21-days as a single dose. LY293111 was administered twice daily continuously by mouth.
Results: Dose limiting toxicity (DLT) of grade 3 diarrhea was seen in two patients with doses of irinotecan 300 mg/m2 IV every 21-days in combination with LY293111 300 mg BID. Subsequently the dose of irinotecan was decreased to 250 mg/m2 IV every 21-days with escalating doses of LY293111. A DLT of grade 3 abdominal pain was seen at dose 600 mg BID of LY293111 with irinotecan 250 mg/m2. The pharmacokinetics (PK) indicated that the administration of LY293111 did not have an effect on the PK of irinotecan or its metabolite SN-38. No responses were seen; seven patients had stable disease of a median duration of 4.4 months (range 2.8–13 months).
Conclusion: The recommended phase II dose of LY293111 is 600 mg orally BID in combination with irinotecan 250 mg/m2 IV every 21-days. Gastrointestinal adverse effects were common but could be well managed.



Similar content being viewed by others
References
Tang DG, Chen YQ, Honn KV (1996) Arachidonate lipoxygenases as essential regulators of cell survival and apoptosis. Proc Natl Acad Sci USA 93:5241–5246
Crooks SW, Stockley RA (1998) Leukotriene B4. Int J Biochem Cell Biol 30(2):173–178
Hagmann W (1997) Lipoxygenase in human tumor cells. Pathol Oncol Res 3(2):83–88
Reich R, Martin GR (1996) Identification of arachidonic acid pathways required for the invasive and metastatic activity of malignant tumor cells. Prostaglandins 51(1):1–17
Bortuzzo C, Hanif R, Kashfi K, Staiano-Coico L, Shiff SJ, Rigas B (1996) The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim Biophys Acta 1300(3):240–246
Mann EL, Spiro JD, Chen LL, Kreutzer DL (1994) Phospholipid metabolite expression by head and neck squamous cell carcinoma. Arch Otolaryngol Head Neck Surg 120(7):763–769
Jackson, WT, Froelich LL, Boyd RJ, Schrementi JP, Saussy DL, Schultz RM, Sawyer JS, Sofia MJ, Herron DK, Goodson T, Snyder DW, Pechous PA, Spaethe SM, Roman CR, Fleisch JH (1999) Pharmacologic actions of the second-generation Leukotriene B4 Receptor Antagonist LY293111: in vitro studies. J Pharmacol Exp Ther 288(1):286–294
Mommers JM, Van Tossum MM, Kooijmans-Otero ME, Parker FL, van de Kerkhof PC (2000) VML 295(LY-293111), a novel LTB4 antagonist, is not effective in the prevention of relapse in psoriasis. Br J Dermatol 142(2):259–266
Evans DJ, Barnes PJ, Spaethe SM, van Alstyne EL, Mitchell MI, O’Connor BJ (1996) Effect of a leukotriene B4 receptor antagonist, LY293111, on allergen induced responses in asthma. Thorax 51(12):1178–1173
Marshall M, Diaz HB, Brozinick J et al (2002) LY293111 inhibits tumor cell growth in vitro through an apparent PPARγ agonist activity. Proc Amer Assoc Cancer Res 43:957 (abstract 4741)
Vamecq J, Latruffe N (1999) Medical significance of peroxisome proliferator-activated receptors. Lancet 354:141–148
Koeffler HP (2003) Peroxisome proliferators-activated receptor γ and cancers. Clin Can Res 9:1–9
Hennig R, Ding X, Tong W, Witt RC, Jovanovic BD, Adrian TE (2004) Effect of LY293111 in combination with gemcitabine in colonic cancer. Cancer Letters 210:41–46
Ding X, Talamonti M, Bell R Jr, Adrian TE (2005) A novel anti-pancreatic cancer agent, LY293111. Anticancer Drugs 16(5):467–473
Zhang W, McQueen T, Schober W, Rassidakis G, Andreeff, M, Konopleva M (2005) Leukotriene B4 receptor inhibitor LY293111 induces cell cycle arrest and apoptosis in human anaplastic large-cell lymphoma cells via JNK phosphorylation. Leukemia 19:1977–1984
Tong W-G, Ding X-Z, Hennig R, Witt RC, Standop J, Pour PM, Adrian TE (2002) Leukotriene B4 receptor antagonist LY293111 inhibits proliferation and induces apoptosis in human pancreatic cancer cell. Clin Cancer Res 8:3232–3242
Schwartz GK, Weitzman A, O’Reilly E, Brail L, de Alwis DP, Cleverly A, Barile-Thiem B, Vinciguerra V, Budman DR (2005) Phase I and pharmacokinetic study of LY293111, an orally bioavailable LTB4 receptor antagonist, in patients with advanced solid tumors. J Clin Oncol 23(23):5365–5373
Rosen LS (1998) Irinotecan in lymphoma, leukemia, and breast, pancreatic, ovarian, and small-cell lung cancers. Oncology suppl 6:103–109
Budman DR, Calabro A (2004) Studies of synergistic and antagonistic combinations of conventional cytotoxic agents with the multiple eicosanoid pathway modulator LY 293111. Anti-Cancer Drugs 15(9):877–881
Armand JP, Extra YM, Catimel G, Abigerges D, Marty M, Clavel M (1996) Rationale for the dosage and schedule of CPT-11(irinotecan) selected for phase II studies, as determined by European phase I studies. Ann Oncol 7(8):837–842
Therasse P, Arbuck SG, Eisenhauer EA, Wander J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors (RECIST guidelines). J Natl Cancer Inst 92:205–216
Abigerges D, Chabot GG, Armand J-P, Herait P, Gouyette A, Gandia D (1995) Phase I and pharmacologic studies of the camptothecin analog irinotecan administered every 3 weeks in cancer patients. J Clin Oncol 13(1):210–221.
Pitot HC, Goldberg RM, Reid JM, Sloan JA, Skaff PA, Erlichman C, Rubin J, Burch PA, Adjei AA, Albers SA, Schaaf LJ, Elfring G, Miller LL (2000) Phase I dose-finding and pharmacokinetic trial of irinotecan hydrochloride (CPT-11) using a once-every-three-week dosing schedule for patients with advanced solid tumor malignancy. Clin Cancer Res 6(6):2236–2244
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baetz, T., Eisenhauer, E., Siu, L. et al. A phase I study of oral LY293111 given daily in combination with irinotecan in patients with solid tumours. Invest New Drugs 25, 217–225 (2007). https://doi.org/10.1007/s10637-006-9021-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-9021-8