Summary
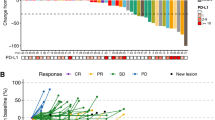
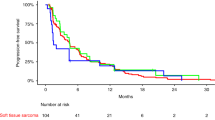
Background/Patients and methods: 16 adult patients with untreated measurable locally advanced or metastatic inoperable soft tissue sarcoma were treated with oral perifosine, a synthetic alkylphospholipid, believed to inhibit MAP kinase (MAP-K), protein kinase C (PKC), Akt and other regulatory proteins. Perifosine was administered orally in cycles for 21 days out of 28. Loading doses were given day 1 each cycle (900 mg cycle 1, 300 mg cycle 2+) and 150 mg daily was given days 2–21 of each cycle. Cycles were repeated until disease progression, unacceptable toxicity or patient refusal. Results: Seventeen patients were enrolled; 16 and 15 were evaluable for toxicity and response, respectively. A total of 30 cycles of perifosine were administered. Most toxic effects were grade 1 or 2 and commonly included nausea, vomiting, diarrhea, and fatigue (≥40%). Hematologic toxicity was generally mild. There were no significant biochemical abnormalities due to the drug reported. There were 4 serious adverse events (SAE)—none of which was related to perifosine. No objective responses were seen; 4 patients had stable disease for 1.3 to 8.2 months and the remainder of the patients had progressive disease.Conclusions: Perifosine when given according to this dosing schedule does not show evidence of activity in a mixed population of adult soft tissue sarcoma patients.
Similar content being viewed by others
References
Bramwell VH, Anderson D, Charette ML, Sarcoma Disease Site G (2003) Doxorubicin-based chemotherapy for the palliative treatment of adult patients with locally advanced or metastatic soft tissue sarcoma. Cochrane Database of Systematic Reviews (3):CD003293
Verma S, Bramwell V (2002) Dose-intensive chemotherapy in advanced adult soft tissue sarcoma. Exp Rev Anticancer Ther 2(2):201–215
Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, Heinrich MC, Tuveson DA, Singer S, Janicek M, Fletcher JA, Silverman SG, Silberman SL, Capdeville R, Kiese B, Peng B, Dimitrijevic S, Druker BJ, Corless C, Fletcher CDM, Joensuu H (2002) Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 347(7):472–480
Maki RG, Awan RA, Dixon RH, Jhanwar S, Antonescu CR (2002) Differential sensitivity to imatinib of 2 patients with metastatic sarcoma arising from dermatofibrosarcoma protuberans. International J Cancer 100(6):623–626
McArthur GA, Demetri GD, van Oosterom A, Heinrich MC, Debiec-Rychter M, Corless CL, Nikolova Z, Dimitrijevic S, Fletcher JA (2005) Molecular and clinical analysis of locally advanced dermatofibrosarcoma protuberans treated with imatinib: Imatinib target exploration consortium study B2225. J Clin Oncol 23(4):866–873
Verweij J, Van Oosterom A, Blay JY, Judson I, Rodenhuis S, Van Der Graaf W, Radford J, Le Cesne A, Hogendoorn PCW, Di Paola ED, Brown M, Nielsen OS (2003) Imatinib mesylate (STI-571 Glivec, Gleevec) is an active agent for gastrointestinal stromal tumours, but does not yield responses in other soft-tissue sarcomas that are unselected for a molecular target: Results from an EORTC Soft Tissue and Bone Sarcoma Group phase II study. Europ J Cancer 39(14):2006–2011
Uberall F, Oberhuber H, Maly K, Zaknun J, Demuth L, Grunicke H (1991) Hexadecylphosphocholine inhibits inositol phosphate formation and protein kinase C activity. Cancer Res 51(3):807–812
Ruiter GA, Zerp SF, Bartelink H, Van Blitterswijk WJ, Verheij M (1999) Alkyl-lysophospholipids activate the SAPK/JNK pathway and enhance radiation-induced apoptosis. Cancer Res 59(10):2457–2463
Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK (2003) Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther 2(11):1093–103
Cuadrado A, Issing W, Fleming TP, Molloy CJ (1994) Uneven distribution of protein kinase C-alpha and -beta isozymes in human sarcomas and carcinomas. J Cell Physiol 159(3):434–440
Issing WJ, Wustrow TP (1991) Overexpression of protein kinase-C-isoenzymes in human tumor cell lines. Laryngo-Rhino-Otologie 70(3):146–150
Unger C: Study D-21266-3040 AOI Phase I study Group. Unpublished data
Unger C: Study D-21266-3087. Weekly enteric coated tablets. AOI Phase I study Group. Unpublished data
Crul M, Rosing H, De Klerk GJ, Dubbelman R, Traiser M, Reichert S, Knebel NG, Schellens JHM, Beijnen JH, Ten Bokkel Huinink WW (2002) Phase I and pharmacological study of daily oral administration of perifosine (D-21266) in patients with advanced solid tumours. Europ J Cancer 38(12):1615–1621
Monga M, Messmann R, Headlee D, Woo EW, Figg WD, Murgo A, Sausville EA: A Phase I trial of oral perifosine in patients with refractory neoplasms: ASCO, 2002, pp #1837
Van Ummersen L, Binger K, Volkman J, Marnocha R, Tutsch K, Kolesar J, Arzoomanian R, Alberti D, Wilding G (2004) A phase I trial of perifosine (NSC 639966) on a loading dose/maintenance dose schedule in patients with advanced cancer. Clin Cancer Res 10(22):7450–7456
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, Van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J National Cancer Inst 92(3):205–216
Zee B, Melnychuk D, Dancey J, Eisenhauer E (1999) Multinomial phase II cancer trials incorporating response and early progression. J Biopharm Statist 9(2):351–363
Freidlin B, Dancey J, Korn EL, Zee B, Eisenhauer E (2002) Multinomial Phase II Trial Designs. J Clin Oncol 20(2):599
Patel V, Lahusen T, Sy T, Sausville EA, Gutkind JS, Senderowicz AM (2002) Perifosine, a novel alkylphospholipid, induces p21(WAF1) expression in squamous carcinoma cells through a p53-independent pathway, leading to loss in cyclin-dependent kinase activity and cell cycle arrest. Cancer Res 62(5):1401–1409
Ruiter GA, Zerp SF, Bartelink H, Van Blitterswijk WJ, Verheij M (2003) Anti-cancer alkyl-lysophospholipids inhibit the phosphatidylinositol 3-kinase-Akt/PKB survival pathway. Anti Cancer Drugs 14(2):167–173
Author information
Authors and Affiliations
Corresponding author
Additional information
Grant Support: Supported by a research grant form the National Cancer Institute of Canada with funds from the Canadian Cancer Society.
Rights and permissions
About this article
Cite this article
Knowling, M., Blackstein, M., Tozer, R. et al. A phase II study of perifosine (D-21226) in patients with previously untreated metastatic or locally advanced soft tissue sarcoma: A National Cancer Institute of Canada Clinical Trials Group trial. Invest New Drugs 24, 435–439 (2006). https://doi.org/10.1007/s10637-006-6406-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-6406-7