Summary
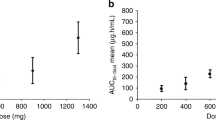
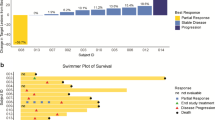
DNA methyltransferases (DNMTs) methylate DNA, promoting local chromatin condensation and consequent repression of gene expression. The purpose of this two-stage phase II trial was to assess the antitumor activity of MG98, a second generation antisense oligodeoxynucleotide inhibitor of human DNMT 1, in patients with metastatic renal carcinoma (MRC). Untreated adult patients with measurable MRC were treated with MG98 at a dose of 360 mg/m2 via 2-h iv infusion twice weekly for three consecutive weeks out of four. The primary endpoint was objective response or absence of progression for at least eight weeks. Pharmacokinetics and DNMT1 mRNA levels in peripheral blood mononuclear cells (PBMCs) were also analyzed at pre-specified intervals. Seventeen eligible patients received a median of two cycles of treatment (range, 1–7), and no objective responses were seen. Nine patients had progressive disease, six had stable disease, and the study was stopped after the first stage. The most common symptomatic toxicities were rigors, fatigue, fever, and nausea. Hematological toxicity was mild. Seven patients treated with prior nephrectomy had grade 3 or 4 elevations in hepatic transaminases. Significantly higher Cmax and AUC(0→inf) values were observed in these patients. No conclusive pattern of decreased DNMT1 activity in PBMCs was detected post MG98 treatment. The lack of objective responses observed may be explained by a lack of target effect or the choice of tumor type. Transaminitis was observed in patients with prior nephrectomy and appeared to be associated with altered drug exposure in these patients.
Similar content being viewed by others
References
Leonhardt H, Cardoso MC (2000) DNA methylation, nuclear structure, gene expression and cancer. J Cell Biochem 35(Supp l):78–83
Merlo A, Herman JG, Mao L, Lee DJ, Gabrielson E, Burger PC, Baylin SB, Sidransky D (1995) 5′ CpG island methylation is associated with transcriptional silencing of the tumor suppressor p16/CDKN2/MTS1 in human cancers. Nature Med 1:686–692
Kautiainen TL, Jones PA (1996) DNA methyltransferase levels in tumorigenic and nontumorigenic cells in culture. J Biol Chem 261:1594–1598
Kausch I, Böhle A (2002) Antisense oligonucleotide therapy in urology. J Urol 168:239–247
Jansen B, Zangemeister-Wittke U (2002) Antisense therapy for cancer—the time of truth. Lancet Oncol 3:672–682
MG98 Investigator's Brochure, MethylGene Inc, July 12, 2001
Bealieu N, Fournel M, MacLeod AR (2001) Antitumor activity of MG98, an antisense oligodeoxynucleotide targeting DNA methyltransferase-1. Clin Cancer Res 7(Supp l):3800s
Davis A, Gelmon K, Siu L, Moore MJ, Britten CD, Mistry N, Klamut H, D'Aloisio S, MacLean M, Wainman N, Ayers D, Firby P, Besterman JM, Reid GK, Eisenhauer EA (2003) Phase I and pharmacologic study of the human DNA methyltranseferase antisense oligodeoxynucleotide MG98 given as a 21-day continuous infusion every 4 weeks. Invest New Drug 21:1–13
Stewart DJ, Donehower RC, Eisenhauer EA, Wainman N, Shah AK, Bonfils C, MacLeod AR, Besterman JM, Reid GK (2003) A phase I and pharmacodynamic study of the DNA methyltransferase 1 inhibitor MG98 administered twice weekly. Ann Oncol 14:766–774
Coppin C, Porzsolt F, Kumpf J, Coldman A, Wilt T (2002) Immunotherapy for advanced renal cell cancer (Cochrane Review). In: The Cochrane Library, Issue 3. Update Software, Oxford
Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R (2001) Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet 22(358):966–970
Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, Caton JR Jr, Munshi N, Crawford ED (2001) Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med 345:1655–1659
Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM, Baylin SB (1994) Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci USA 91:9700–9704
Dreijerink K, Braga E, Kuzmin I Geil L, Duh FM, Angeloni D, Zbar B, Lerman MI, Stanbridge EJ, Minna JD, Protopopov A, Li J, Kashuba V, Klein G, Zabarovsky ER (2001) The candidate tumor suppressor gene, RASSF1A, from human chromosome 3p21.3 is involved in kidney tumorigenesis. Proc Natl Acad Sci USA 98:7504–7509
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 92:205–216
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41
Chi KN, Gleave ME, Klasa R, Murray N, Bryce C, Lopes de Menezes DE, D'Aloisio S, Tolcher AW (2001) A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clin Cancer Res 7:3920–3927
Morris MJ, Tong WP, Cordon-Cardo C, Drobnjak M, Kelly WK, Slovin SF, Terry KL, Siedlecki K, Swanson P, Rafi M, DiPaola RS, Rosen N, Scher HI (2002) Phase I trial of BCL-2 antisense oligonucleotide (G3139) administered by continuous infusion in patients with advanced cancer. Clin Cancer Res 8:679–683
Tolcher AW, Kuhn J, Schwartz G, Patnaik A, Hammond LA, Thompson I, Fingert H, Bushnell D, Malik S, Kreisberg J, Izbicka E, Smetzer L, Rowinsky EK (2004) A phase I pharmacokinetic and biological correlative study of oblimersen sodium (Genasense, G3139), and antisense oligonucleotide to the Bcl-2 mRNA, and of docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 10:5048–5057
Rudin CM, Marshall JL, Huang CH, Kindler HL, Zhang C, Kumar D, Gokhale PC, Steinberg J, Wanasaki S, Kasid UN, Ratain MJ (2004) Delivery of a liposomal c-raf-1 antisense oligonucleotide by weekly bolus dosing in patients with advanced solid tumors: a phase I study. Clin Cancer Res 10:7244–7251
Tolcher AW, Chi K, Kuhn J, Gleave M, Patnaik A, Takimoto C, Schwartz G, Thompson I, Berg K, D'Aloisio S, Murray N, Frankel SR, Izbicka E, Rowinsky EK (2005) A phase II, pharmacokinetic, and biological correlative study of oblimersen sodium and docetaxel in patients with hormone-refractory prostate cancer. Clin Cancer Res 11:3854–3861
Gleave ME, Elhilali M, Fradet Y, Davis I, Venner P, Saad F, Klotz LH, Moore MJ, Paton V, Bajamonde A (1998) Interferon gamma-1b compared with placebo in metastatic renal-cell carcinoma. N Engl J Med 338:1265–1271
Medical Research Council Renal Cancer Collaborators (1999) Interferon-α and survival in metastatic renal cell carcinoma: early results of a randomised controlled trial. Lancet 353: 14–17
Pyrhonen S, Salminen E, Ruutu M, Lehtonen T, Nurmi M, Tammela T, Juusela H, Rintala E, Hietanen P, Kellokumpu-Lehtinen PL (1999) Prospective randomized trial of interferon alfa-2a plus vinblastine versus vinblastine alone in patients with advanced renal cell cancer. J Clin Oncol 17:2859–2867
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Winquist, E., Knox, J., Ayoub, JP. et al. Phase II trial of DNA methyltransferase 1 inhibition with the antisense oligonucleotide MG98 in patients with metastatic renal carcinoma: A National Cancer Institute of Canada Clinical Trials Group investigational new drug study. Invest New Drugs 24, 159–167 (2006). https://doi.org/10.1007/s10637-006-5938-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-006-5938-1