Abstract
Purpose
To investigate the relationship between adaptation time and the parameters of electroretinography (ERG) and pupillography in healthy subjects.
Methods
Forty-six eyes of 23 healthy women (mean age 21.7 years) were enrolled. ERG and pupillography were tested in each of the right and left 23 eyes, respectively. ERG with a skin electrode was used to determine amplitude and implicit time by the records of rod-, flash-, cone-, and flicker-responses with white light (0.01–30 cd s/m2). Infrared pupillography was used to record the pupillary light reflex to 1-s stimulation of red light (100 cd/m2). Cone- and flicker- (rod-, flash- and pupil) responses were recorded after light (dark) adaptation at 1, 5, 10, 15, and 20 min.
Results
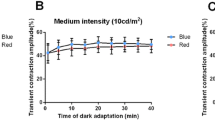
Amplitude (µV) was significantly different between 1 min and ≥ 5 or ≥ 10 min after adaptation in b-wave of cone- or rod-response, respectively. Implicit time (ms) differed significantly between 1 min and ≥ 5 min after adaptation with b-wave of cone- and rod-response. There were significant differences between 1 min and ≥ 10 or ≥ 5 min after dark adaptation in parameter of minimum pupil diameter (mm) or constriction rate (%), respectively.
Conclusions
Cone-driven ERG can be recorded, even in 5 min of light adaptation time without any special light condition, whereas rod-driven ERG and pupillary response results can be obtained in 10 min or longer of dark adaptation time in complete darkness.





Similar content being viewed by others
References
Hecht S (1920) The dark adaptation of the human eye. J Gen Physiol 2(5):499–517 PMID: 19871826
Crawford BH (1947) Visual adaptation in relation to brief conditioning stimuli. Proc R Soc Lond B Biol Sci 134(875):283–302 PMID: 20292379
Wald G, Clark AB (1937) Visual adaptation and chemistry of the rods. J Gen Physiol 21(1):93–105 PMID: 19873041
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV Standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12. https://doi.org/10.1007/s10633-01409473-7
Hamilton R, Graham K (2016) Effect of shorter dark adaptation on ISCEV standard DA 0.01 and DA 3 skin ERGs in healthy adults. Doc Ophthalmol 133(1):11–19. https://doi.org/10.1007/s10633-016-9554-x
Wang B, Shen C, Zhang L, Qi L, Yao L, Chen J, Yang G, Chen T, Zhan Z (2015) Dark adaptation-induced changes in rod, cone and intrinsically photosensitive retinal ganglion cell (ipRGC) sensitivity differentially affect the pupil light response (PLR). Graefes Arch Clin Exp Ophthalmol 253(11):1997–2005. https://doi.org/10.1007/s00417-015-3137-5
Fotiou F, Fountoulakis KN, Goulas A, Alexopoulos L, Palikaras A (2000) Automated standardized pupillometry with optical method for purposes of clinical practice and research. Clin Physiol 20(5):336–347
Schnitzler E-M, Baumeister M, Kohnen T (2000) Scotopic measurement of normal pupils: colvard versus video vision analyzer infrared pupillometer. J Cataract Refract Surg 26(6):859–866
Bradley JC, Bentley KC, Mughal AI, Bodhireddy H, Young RS, Brown SM (2010) The effect of gender and iris color on the dark-adapted pupil diameter. J Ocul Pharmacol Ther 26(4):335–340. https://doi.org/10.1089/jop.2010.0061
Lorenz B, Strohmayr E, Zahn S, Friedburg C, Kramer M, Preising M, Stieger K (2012) Chromatic pupillometry dissects function of the three different light-sensitive retinal cell populations in RPE65 deficiency. Invest Ophthalmol Vis Sci 53(9):5641–5652. https://doi.org/10.1167/iovs.12-9974
Bremner FD (2012) Pupillometric evaluation of the dynamics of the pupillary response to a brief light stimulus in healthy subjects. Invest Ophthalmol Vis Sci 53(11):7343–7347. https://doi.org/10.1167/iovs.12-10881
Traustason S, Brondsted AE, Sander B, Lund-Andersen H (2016) Pupillary response to direct and consensual chromatic light stimuli. Acta Ophthalmol 94(1):65–69. https://doi.org/10.1111/aos.12894
Satou T, Ishikawa H, Asakawa K, Goseki T, Shimizu K (2017) Effects of ripasudil hydrochloride hydrate instillation on pupil dynamics. Curr Eye Res 42(1):54–57. https://doi.org/10.3109/02713683.2016.1148740
Yuhas PT, Shorter PD, McDaniel CE, Earley MJ, Hartwick AT (2017) Blue and red light-evoked pupil responses in photophobic subjects with TBI. Optom Vis Sci 94(1):108–117. https://doi.org/10.1097/OPX.0000000000000934
Lisowska J, Lisowski L, Kelbsch C, Maeda F, Richter P, Kohl S, Zobor D, Strasser T, Stingl K, Zrenner E, Peters T, Wilhelm H, Fischer MD, Wilhelm B, RD-CURE Consortium (2017) Development of a chromatic pupillography protocol for the first gene therapy trial in patients with CNGA3-linked achromatopsia. Invest Ophthalmol Vis Sci 58(2):1274–1282. https://doi.org/10.1167/iovs.16-20505
Lawlor M, Quartilho A, Bunce C, Nathwani N, Dowse E, Kamal D, Gazzard G (2017) Patients with normal tension glaucoma have relative sparing of the relative afferent pupillary defect compared to those with open angle glaucoma and elevated intraocular pressure. Invest Ophthalmol Vis Sci 58(12):5237–5241. https://doi.org/10.1167/iovs.17-21688
Crippa SV, Pedrosa Domellöf F, Kawasaki A (2018) Chromatic pupillometry in children. Front Neurol 9:669. https://doi.org/10.3389/fneur.2018.00669
Normann RA, Werblin FS (1974) Control of retinal sensitivity. I. Light and dark adaptation of vertebrate rods and cones. J Gen Physiol 63(1):37–61 PMID: 4359063
Baylor DA, Nunn BJ, Schnapf JL (1984) The photocurrent, noise and spectral sensitivity of rods of the monkey Macaca fascicularis. J Physiol 357:575–607
Pugh E, Altman J (1988) Phototransduction. A role for calcium in adaptation. Nature 334(6177):16–17. https://doi.org/10.1038/334016a0
Aguilar M, Stiles WS (1954) Saturation of the rod mechanism of the retina at high levels of stimulation. Opt Acta 1:59–65
Baker HD (1949) The course of foveal light adaptation measured by the threshold intensity increment. J Opt Soc Am 39(2):172–179
Yanagisawa Y, Yoshino H, Ishikawa S, Miyata M (2017) Chemical sensitivity and sick-building syndrome. CRC Press, Boca Raton
Tsujisawa I, Mukuno K, Ishikawa S (1989) The pupillary change in the course of brain death. J Auton Nerv Syst 26:63–70 (in Japanese)
Lowenstein O (1955) Pupillary reflex shapes and topical clinical diagnosis. Neurology 5(9):631–644 PMID: 13253831
Gamlin PD, McDougal DH, Pokorny J, Smith VC, Yau KW, Dacey DM (2007) Human and macaque pupil responses driven by melanopsin-containing retinal ganglion cells. Vis Res 47(7):946–954. https://doi.org/10.1016/j.visres.2006.12.015
Kardon R, Anderson SC, Damarjian TG, Grace EM, Stone E, Kawasaki A (2009) Chromatic pupil responses: preferential activation of the melanopsin-mediated versus outer photoreceptor-mediated pupil light reflex. Ophthalmology 116(8):1564–1573. https://doi.org/10.1016/j.ophtha.2009.02.007
Asakawa K, Ishikawa H, Uga S, Mashimo K, Shimizu K, Kondo M, Terasaki H (2015) Functional and morphological study of retinal photoreceptor cell degeneration in transgenic rabbits with a Pro347Leu rhodopsin mutation. Jpn J Ophthalmol 59(5):353–363. https://doi.org/10.1007/s10384-015-0400-6
Acknowledgements
The authors thank Yuuki Nakayama, Yousuke Horiuchi of Uni-hite corporation, for technical assistance with data collection; Robert E. Brandt, Founder, CEO, and CME, of MedEd Japan, for editing and formatting the manuscript. This study was supported by a grant from Kitasato University School of Allied Health Sciences Grant in-Aid for Research Project, Grant Number 2018-1041.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Statement of human rights
All research procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all subjects in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asakawa, K., Ito, A., Kobayashi, H. et al. Adaptation time, electroretinography, and pupillography in healthy subjects. Doc Ophthalmol 139, 33–44 (2019). https://doi.org/10.1007/s10633-019-09693-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-019-09693-8