Abstract
Purpose
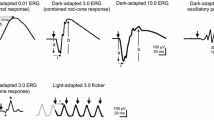
The full-field electroretinogram (ff-ERG) is a widely used clinical tool to evaluate generalized retinal function by recording electrical potentials generated by the cells in the retina in response to flash stimuli and requires mydriasis. The purpose of this study was to determine the intra-visit reliability and diagnostic capability of a handheld, mydriasis-free ERG, RETeval (LKC Technologies, Gaithersburg, MD, USA), in comparison with the standard clinical ff-ERG by measuring responses recommended by the International Society for Clinical Electrophysiology of Vision (ISCEV).
Methods
This prospective, cross-sectional study included 35 patients recruited at the Hospital for Sick Children (median age = 17, range 11 months–69 years) who had undergone a clinical ff-ERG according to ISCEV standards. For RETeval (n = 35), pupils were undilated in most (n = 29) and sensor strip electrodes were placed under the inferior orbital rim. Stimulus settings on RETeval were equivalent to those used in the clinical ERG. Fifty-seven control participants (median age = 22, range 8–65 years) underwent undilated RETeval ERG to establish standard values for comparison. Patient waveform components with amplitudes < 5th percentile, or implicit times > 95th percentile of normal relative to control data were classified as abnormal for the RETeval system.
Results
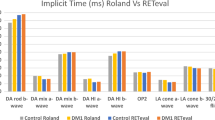
The RETeval system demonstrated a high degree of within-visit reliability for amplitudes (ICC = 0.82) and moderate reliability for implicit times (ICC = 0.53). Cohen’s Kappa analysis revealed a substantial level of agreement between the diagnostic capability of RETeval in comparison with clinical ff-ERG (k = 0.82), with a sensitivity and specificity of 1.00 and 0.82, respectively. Pearson’s correlations for clinical ERG versus RETeval demonstrated a positive correlation for amplitudes across the rod (r = 0.65) and cone (r = 0.74) ERG waveforms. Bland–Altman plots showed no bias between the mean differences across all amplitude and implicit time parameters of the two systems.
Conclusions
The present study demonstrated that RETeval is a reliable tool with reasonable accuracy in comparison with the clinical ERG. The portable nature of RETeval system enables its incorporation at resource-limited centers where the ff-ERG is not readily available. The avoidance of sedation and pupillary dilation are added advantages of RETeval ERG.





Similar content being viewed by others
References
McCulloch DL, Marmor MF, Brigell MG, Hamilton R, Holder GE, Tzekov R, Bach M (2015) ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol 130(1):1–12
Barber C (1994) Electrodes and the recording of the human electroretinogram (ERG). Int J Psychophysiol 16(2–3):131–136
Kato K, Kondo M, Sugimoto M, Ikesugi K, Matsubara H (2015) Effect of pupil size on flicker ERGs recorded with RETeval system: new mydriasis-free full-field ERG system. Invest Ophthalmol Vis Sci 56(6):3684–3690
Kato K, Kondo M, Nagashima R, Sugawara A, Sugimoto M, Matsubara H, McCulloch DL, Ikesugi K (2017) Factors affecting mydriasis-free flicker ERGs recorded with real-time correction for retinal illuminance: study of 150 young healthy subjects. Invest Ophthalmol Vis Sci 58(12):5280–5286
Bradshaw K, Hansen R, Fulton A (2004) Comparison of ERGs recorded with skin and corneal-contact electrodes in normal children and adults. Doc Ophthalmol 109(1):43–55
Maa AY, Feuer WJ, Davis CQ, Pillow EK, Brown TD, Caywood RM, Chasan JE, Fransen SR (2016) A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J Diabetes Complicat 30(3):524–532
Fukuo M, Kondo M, Hirose A, Fukushima H, Ikesugi K, Sugimoto M, Kato K, Uchigata Y, Kitano S (2016) Screening for diabetic retinopathy using new mydriasis-free, full-field flicker ERG recording device. Sci Rep 6:36591
Nakamura N, Fujinami K, Mizuno Y, Noda T, Tsunoda K (2016) Evaluation of cone function by a handheld non-mydriatic flicker electroretinogram device. Clin Ophthalmol (Auckland, NZ) 10:1175
Kurtenbach A, Kramer S, Strasser T, Zrenner E, Langrová H (2017) The importance of electrode position in visual electrophysiology. Doc Ophthalmol 134(2):129–134
Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346:1085–1087
Asakawa K, Amino K, Iwase M, Kusayanagi Y, Nakamura A, Suzuki R, Yuuki T, Ishikawa H (2017) New mydriasis-free electroretinogram recorded with skin electrodes in healthy subjects. BioMed Res Int. https://doi.org/10.1155/2017/8539747
Westall CA, Panton CM, Levin AV (1999) Time courses for maturation of electroretinogram responses from infancy to adulthood. Doc Ophthalmol 96(4):355–379
Birch DG, Anderson JL (1992) Standardized full-field electroretinography: normal values and their variation with age. Arch Ophthalmol 110(11):1571–1576
Karpe G, Rickenbach K, Thomasson S (1950) The clinical electroretinogram. I. The normal electroretinogram above fifty years of age. Acta Ophthalmol 28(3):301
Fulton AB, Hansen RM, Westall CA (2003) Development of ERG responses: the ISCEV rod, maximal and cone responses in normal subjects. Doc Ophthalmol 107(3):235–241
Al-Otaibi H, Al-Otaibi MD, Khandekar R, Souru C, Al-Abdullah AA, Al-Dhibi H, Stone DU, Kozak I (2017) Validity, usefulness and cost of RETeval system for diabetic retinopathy screening. Transl Vis Sci Technol 6(3):3
Golshani C, Brodie SE (2018) Quantitative calibration of sensor strip ERG electrodes. Poster presented at the 2018 annual meeting of the association for research in vision and ophthalmology, Honolulu, HI
Leguire LE, Rogers GL (1985) Pattern electroretinogram: use of noncorneal skin electrodes. Vis Res 25(6):867–870
Miura G, Nakamura Y, Sato E, Yamamoto S (2016) Effects of cataracts on flicker electroretinograms recorded with RETeval™ system: new mydriasis-free ERG device. BMC Ophthalmol 16(1):22
Acknowledgements
We thank Dr. Stacey Chong for her assistance with clinical testing and Dr. Annie Dupuis for her input regarding statistical analysis. We would also like to thank Mary Yang, Vishruti Dalal, and Aranie Muraleetharan for their assistance with data collection. This work was supported by the Vision Science Research program at the University of Toronto (Xiang Ji), SickKids Ophthalmology Research Fund (Dr. Carol Westall), and Foundation Fighting Blindness USA Career Development Award (Dr. Ajoy Vincent) (Grant No. CD-CL-0617-0727-HSC). LKC Technologies, Inc. provided the RETeval device and some sensor strips electrodes used in this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The author declares that they have no conflict of interest.
Statement of human rights
All research procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Statement on the welfare of animals
This article does not contain any studies with animals.
Informed consent
Informed consent was obtained from all participants included in the study.
Rights and permissions
About this article
Cite this article
Liu, H., Ji, X., Dhaliwal, S. et al. Evaluation of light- and dark-adapted ERGs using a mydriasis-free, portable system: clinical classifications and normative data. Doc Ophthalmol 137, 169–181 (2018). https://doi.org/10.1007/s10633-018-9660-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10633-018-9660-z