Abstract
The inflammatory bowel diseases (IBD) are complex immune-mediated inflammatory diseases that are associated with significant morbidity around the world. As our understanding of IBD, and other immune-mediated inflammatory diseases, advances the number of therapeutic targets has increased which has rapidly driven the development and introduction of new therapies. While these new therapies have shown promise they come with the significant drawback of high costs. For many IBD patients around the world the cost of newer therapies is prohibitive which means treating clinicians often need to turn to optimising simpler, older, and inexpensive medications. The concept of optimising well established cheaper medications is not unique to the management of IBD as health systems all over the world look to reduce costs while simultaneously improving patient outcomes. Despite thiopurines being used in the management IBD for over 60 years, many clinicians are still hesitant to use them due to perceptions around limited efficacy and poor tolerance. One method identified to potentially increase utilisation of thiopurines involves the coadministration of allopurinol. In this review we will explore the history, pharmacology, recent studies and give recommendations for the utilisation of the usual duo of azathioprine combined with allopurinol.
Similar content being viewed by others
Why It’s Important to Review This Topic
The inflammatory bowel diseases (IBD) continue to represent complex diseases with significant morbidity and quality of life impairment. These immune-mediated inflammatory diseases require the use of immunomodulators and/or biologic agents in a significant proportion of patients. Many treatments for IBD are shared across numerous indications from autoimmune diseases through to organ transplantation. Over the last 50 years there has been an increase in the medications that have become available for the treatment of IBD. However, as the understanding of IBD increases and new treatment targets are identified, the medications being developed are expensive due to high production costs [1,2,3,4]. With health care costs rising globally, many health systems may benefit from optimising older, simpler and cheaper therapies [5,6,7]. Thiopurines, including azathioprine, mercaptopurine and thioguanine, particularly when optimised using therapeutic drug monitoring and/or concomitant allopurinol, are one such class of medications.
While thiopurines have not been able to demonstrate equivalent effectiveness when compared to biologics, such as anti- tumour necrosis factor (anti-TNF) agents, used alone or in combination with immunomodulators, they do have several advantages [7,8,9,10,11]. The most obvious benefits of thiopurines are reduced cost, ease of administration and simpler storage. As a result, thiopurines still have an important role in IBD management globally. The optimisation of thiopurines is associated with increased rates of clinical and endoscopic remission [12, 13]. Intuitively this should translate to reduced rates of escalation of therapy to other classes, including biologics, with resulting cost-savings, although we acknowledge that this perception is not supported by results from well-designed prospective studies.
While in many countries the combination of azathioprine and allopurinol costs patients approximately 1% of their countries GDP/Capita, biologic therapy can cost patients in wealthy countries more than 100% of their countries GDP/Capita with the relative price being even higher in developing countries [1, 3, 6, 14, 15]. Fortunately, the cost of biologics is falling, in some cases by 90%, with the utilisation of biosimilars [16, 17]. However, despite this reduction in costs biologic therapies remain expensive compared to other therapies which may limit their availability, especially in developing countries [10, 11, 18, 19].
In recent years the tolerability and safety of thiopurines has been questioned, which has limited their use in some jurisdictions, and North America in particular [1]. Fortunately, as is the focus of this review, the combination of allopurinol with azathioprine presents a therapeutic option for some patients who experience intolerance or side effects of azathioprine monotherapy. This includes patients who require thiopurines in combination therapy with biologics, in particular with anti-TNF agents.
History of Azathioprine and Allopurinol
The purine analogues ‘thiopurines’ were first created by future Nobel laureates George Hitchings and Gertrude Elion in the 1940s as scientists strived to discover new antibiotic medications around the time of World War Two [20]. In their laboratory they revealed that the thiopurines were not useful antibiotics but were noted to be effective against leukemia cells in mice, however toxicity limited their use. By 1951, further refining of thiopurines led to the creation of 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG) which were subsequently used to treat children with acute leukemias who up until that point only had corticosteroids and methotrexate available as treatments. The Food and Drug Administration of the United States of America approved thiopurine medications for use in 1953 [21].
6-TG, which had been synthesised before 6-MP, was more difficult to manufacture and more toxic, leading to 6-MP being favoured. Numerous attempts were made to modify 6-MP to make it more target-specific and better tolerated. The most successful compound, that was created in 1957, was 6-(3-methyl-5-nitroimidazol-4-yl) sulfanyl-7H-purine which would later be named azathioprine. Azathioprine and 6-MP went on to be used extensively in transplantation and leukaemia before first being used in IBD in 1962 where they were used to maintain remission in patients with ulcerative colitis [22]. Since the 1960s thiopurines have continued to be used in the management of IBD and numerous other medical conditions [23, 24].
In parallel to the work on thiopurines, numerous xanthine oxidase inhibitors were being used in laboratory work to modify concentrations of naturally occurring purines. Eventually the desire to use a xanthine oxidase inhibitor for in vivo human experiments led to the development of a minimally toxic medication, 4-hydroxypyrazolo (3,4-d) pyrimidine, which would subsequently be named allopurinol. Allopurinol was trialled to improve the efficacy and reduce the toxicity of thiopurines used to treat leukemias. The initial studies didn’t demonstrate significant benefits and the use of the allopurinol for this purpose was discontinued [25]. Interest in allopurinol re-emerged in 1965 when it was recognised that it could be used to reduce uric acid levels in patients with gout and tumour related hyperuricemia [20, 26].
It wasn’t until 1993 that the azathioprine-allopurinol combination was utilised again when it was shown to improve graft survival for patients who had undergone renal transplantation [27]. While thiopurines have been used in IBD since 1962, interest in the combination of azathioprine and allopurinol re-emerged in 2005 when the beneficial effects of this combination on thiopurine metabolism and tolerance was demonstrated in patients experiencing inefficacy or adverse effects to thiopurines [28]. This work was replicated by numerous groups, leading to azathioprine and allopurinol currently being routinely used in IBD management globally. More recently its use has expanded to other gastrointestinal indications, including autoimmune hepatitis [29, 30].
Azathioprine: Indications in IBD
In 2022, azathioprine remains a commonly used treatment in IBD. Thiopurines are indicated as steroid-sparing maintenance agents in both Crohn’s disease and ulcerative colitis, but not as induction agents due to their slow onset of action [26]. They are efficacious in the prevention of Crohn’s disease post-operatively, although probably less so than anti-TNF agents [31,32,33,34]. In fistulising Crohn’s disease thiopurines are only recommended for use in combination with an anti-TNF as they are ineffective when used as monotherapy in this setting [31,32,33,34].
Azathioprine: Mechanism of Action in IBD
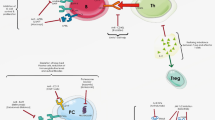
The mechanism of action of azathioprine is still incompletely understood and is likely multifactorial (see Fig. 1). The intracellular metabolites of azathioprine, in particular 6-thioguanine nucleotides (6-TGN), are the most active moieties. Due to their similarity to DNA guanines, 6-TGN are incorporated into cellular DNA particularly in the target cell lines of myeloid lineage. This incorporation into DNA at appropriate frequency can lead to a decreased stability of DNA which is amplified during rapid replication which in turn results in cell death [35, 36]. Another mechanism labelled purine starvation involves alkylating of thiol groups leading to impaired nucleic acid biosynthesis which leads to interferences with DNA replication. Additionally, 6-methylmercaptopurine ribonucleotides (6- MMPR), formed by the action of thiopurine methyltransferase (TPMT) on the thiopurine metabolite 6-thio inosine monophosphate (6-TIMP), may inhibit de novo purine synthesis by blocking phosphoribosylpyrophosphate amido-transferase which would also result in impaired DNA replication [37].
Thiopurine metabolism. AZA azathioprine, 6-MP 6-mercaptopurine, TPMT thiopurine S-methyltransferase, XO xanthine oxidase, 6-TA 6-thiouric acid, 6-TX 6-thioxanthine, HPRT hypoxanthine guanine phosphoribosyltransferase, 6-TIMP 6-thioinosine monophosphate, 6-MeTIMP 6-methylthioinosine monophosphate, IMPDH inosine-5-monophosphate dehydrogenase, 6-MMPR 6-methylmercaptopurine ribonucleotides, 6-TIDP thioinosine diphosphate, 6-TITP thioinosine triphosphate, 6-meTITP methylthioinosine triphosphate, ITPAse inosine triphosphate pyrophosphohydrolase, 6-TXMP thioxanthine monophosphate, GMPS guanidine-5-monophosphate synthetase, 6-TGMP thioguanine monophosphate, 6-TGDP thioguanine diphosphate, 6-TGTP thioguanine triphosphate, 6-TGN thioguanine nucleotides, NUDT15 Nudix hydrolase 15, Rac1 Rac Family Small GTPase 1, I-CAM intercellular adhesion molecule 1, V-CAM vascular cell adhesion protein 1. Modified with permission from Nguyen et al. [4]
A further important mechanism of action involves 6-TGN inhibiting the Rac1 pathway leading to downregulation of CD28, ICAM-1 and VCAM-1. Downregulation of CD28 results in reduced T-cell activation via the T-cell receptor (CD3) costimulatory ligand pathway while reduction in the ICAM-1 and VCAM-1 reduce leukocyte migration into inflammatory sites [38,39,40]. These changes favour immune regulation/tolerance rather than autoimmunity against self-antigens.
Azathioprine: Metabolism, Dosing and Monitoring
Azathioprine has a complex metabolism, leading to variable response and metabolite profiles in individual patients and the need to personalise dosage. Azathioprine is most often prescribed at a dose of 2–2.5 mg/kg orally once daily. Oral azathioprine has a bioavailability of approximately 60% [41] with its absorbance reduced when consumed with food. Azathioprine is excreted as its various metabolites primarily by the kidneys. Once absorbed azathioprine is metabolised in the liver by glutathione-S-transferase into 6-MP and methyl-nitrothioimidazole. Methyl-nitrothioimidazole has minor immunosuppressive effects but causes intolerance in a considerable number of patients who are able to be switched to 6MP successfully [42,43,44].
6-MP is taken up by the target white blood cell progenitors, particularly the myeloid lineage, in the bone marrow. Once inside leukocytes 6-MP is subject to four competing pathways of metabolism (see Fig. 1) [45, 46]. Xanthine oxidase (XO) oxidizes 6-MP to produce the inactive metabolite 6-thiouric acid. Aldehyde oxidase oxidises 6-MP to 6-thioxanthine (6-TX) which may inhibit TPMT. Hypoxanthine guanine phosphoribosyltransferase followed by inosine-5-monophosphate dehydrogenase and finally guanidine-5-monophosphate synthetase coverts 6-MP to the 6-TGN. The 6-TGN, 6-thioguanine monophosphate, 6-thioguanine diphosphate and 6-thioguanine triphosphate, are the primary metabolites that exerts the immunosuppressive action of azathioprine, of which 6-thioguanine triphosphate is known to be the most active particularly in the inhibition of Rac1 [38]. The final competitive pathway for 6-MP involves the action of TPMT which leads to the creation of hepatotoxic 6-MMPR. The variable activity of these enzymes in an individual leads to the specific profile of metabolites produced.
Of the numerous enzymes involved in the metabolism of azathioprine it is the action of three that are considered most important; TPMT, Nudix Hydrolyase 15 (NUDT15) and XO which is inhibited by allopurinol. Functional activity of TPMT is genetically determined with population studies showing its activity has a trimodal distribution [47]. 89% of the population possesses normal or high activity (homozygous), 11% have intermediate activity (heterozygotes) and 0.3% have low or absent enzyme activity [48]. Up to 20% of the population preferentially create 6-MMPR through overactivity of the TPMT pathway and they are known as hyper-methylators or ‘shunters’ [49]. Patients with low or absent TPMT activity are at risk of bone marrow suppression from the high levels of 6-TGN relative to the dose received [46, 47]. TPMT testing, by phenotype or genotype, is now recommended in all patients prior to commencing a thiopurine [8, 47]. More recently, polymorphisms of Nudix NUDT15 have also been associated with myelotoxicity, especially in Asian ethnicities. Accumulation of 6- thioguanosine triphosphate, which is normally inactivated by wildtype NUDT15, is believed to be the cause of this toxicity [50,51,52].
Due to the complex and highly variable metabolism of thiopurines it was identified that the use of therapeutic monitoring could be employed to optimise both safety and efficacy [53, 54]. Initially monitoring was limited to plasma 6-MP and urinary excretion of 6-thiouracic acid [20]. Measuring thiopurine metabolites in myeloid cells proved to be challenging however it was discovered that thiopurines metabolites are absorbed into red blood cells (RBC) and therefore metabolite measurement using RBC levels provided a practical surrogate for myeloid metabolite levels [55]. Numerous studies have shown that a red blood cell 6-TGN levels of greater than 235 pmol/8 × 108 RBC are associated with significantly higher pooled odds ratio [OR] (OR 3.15 95% CI 2.41–4.11 p value ≤ 0.0001) of clinical response to thiopurine therapy [12, 56, 57]. Higher 6-TGN levels of around 400 pmol/8 × 108 RBC have been associated with the harder endpoint of endoscopic healing [13, 58], while levels greater than 450 pmol/8 × 108 RBC have correlated with increased toxicity and minimal further therapeutic gain [12, 57]. Studies have also indicated that red blood cell 6-MMPR levels of greater than 5,700 pmol/8 × 108 RBC are associated with an increased risk of dose limiting hepatotoxicity [57]. It must be acknowledged that most data supporting measurement of thiopurine metabolites comes from retrospective studies. There have been two prospective randomized controlled trials comparing weight-based versus metabolite-based thiopurine dosing. The first study in 2007 concluded that metabolite monitoring did not offer benefit, however it was a small trial and the average 6-TGN levels were below 300 pmol/8 × 108 RBC in both groups [59]. The second trial in 2013 was terminated early due to slow recruitment, however, analyses of the incomplete data suggested trends to improvement in patients with a therapeutic 6-TGN level (> 250 pmol/8 × 108 RBC) [60]. A guide to the interpretation of thiopurine metabolites is provided in Table 1.
Azathioprine: Adverse Effects
Azathioprine is discontinued in patients due to side effects in up to 20–40% of patients with most occurring in the first three months [61,62,63]. Adverse events can be divided into idiosyncratic/allergic reactions, dose (metabolite) dependent reactions and malignancies. Idiosyncratic/allergic reactions include gastrointestinal toxicity, skin toxicity, pancreatitis, constitutional symptoms, and drug induced hypersensitivity reactions. Changing a patient from azathioprine to 6-MP can overcome intolerances in up 50% of cases, consistent with methyl-nitrothioimidazole being the cause of intolerance in these patients [64]. Rat models have suggested that the generation of reactive oxygen species could be another reason for azathioprine toxicity that might not be explained by measurements of metabolites [65].
Dose (and metabolite)-dependent adverse events include myelosuppression, hepatotoxicity, and infections [63, 66, 67]. It is suspected that approximately 20% of patients commenced on azathioprine will experience adverse effects due to toxic levels of the metabolites 6-MMPR (> 5,700 pmol/8 × 108 RBC) and/or 6-TGN (> 450 pmol/8 × 108 RBC) [49]. The increased risk of malignancies in patients with IBD treated with thiopurines may include leukaemia, lymphoma, non-melanoma skin cancer, urothelial and cervical cancer [68,69,70,71]. While the association with non-melanoma skin cancer (hazard ratio 1.4–4.3) and lymphoma (hazard ratio 2.2–5.3) is consistent across numerous studies, the risks of other malignancies, particularly compared to patients treated with biologics, remains less definite [72,73,74,75].
Trials Examining the Use of Azathioprine and Allopurinol in IBD
With respect to retrospective studies, there are numerous groups that have published about the efficacy and safety of combination azathioprine and allopurinol use. All these groups demonstrated that combination azathioprine and allopurinol was useful, safe and effective in the management of IBD [76,77,78,79,80,81]. These studies reported a lower level of cessation for the combination therapy groups when compared to the azathioprine monotherapy groups as well as improved efficacy over monotherapy alone. The dosage of allopurinol used in these studies varied between 100 and 300 mg with 100 mg being the most common dose used. Some groups demonstrated that patients who experienced LFT derangement even after the switch from azathioprine monotherapy to combination azathioprine and allopurinol could have their LFT derangement normalised with an increased dose of allopurinol, from 100 mg to 200–300 mg daily, in almost all cases [76, 79, 81].
There have been four prospective trials that have looked at azathioprine used in combination with allopurinol for the treatment of IBD. The first study, reported in 2013, was a small 11-patient prospective non-randomised study from Switzerland that reported sufficient safety and efficacy despite its small numbers [82]. The second and fourth studies, reported by a Danish group in 2016 and 2022 [58, 83], randomised patients to receive azathioprine monotherapy or combination low-dose azathioprine and allopurinol therapy. The third study, published in 2018 by an Australian group, [84] randomised patients by the dose of allopurinol that was used in combination with an adjusted dose of azathioprine.
In a 2016 pilot study, Kiszka-Kanowitz et al. [83] randomised 46 thiopurine naïve patients with IBD to either azathioprine monotherapy or a combination of low dose azathioprine and allopurinol. After 24 weeks a significant proportion, 69.6%, of the patients treated with combination azathioprine and allopurinol were in clinical steroid free remission compared to 34.7% of the patients treated with azathioprine monotherapy (Relative Risk [RR], 2.10 [95% CI 1.07–4.11]). The study also reported that in the azathioprine group, 47.8% of the patients had to cease the medication due to adverse events compared to 30.4% of the patients in the combination azathioprine and allopurinol group (RR, 1.47 [95% CI 0.76–2.85]).
In 2022 Kiszka-Kanowitz et al. [58] published the findings of their follow up study in 89 patients with ulcerative colitis who were thiopurine naïve and had achieved remission with either steroids or infliximab, and were randomised to receive first-line azathioprine monotherapy or a combination of low dose azathioprine and allopurinol. After 52 weeks, 43% of patients treated with low dose azathioprine and allopurinol achieved remission in comparison with 21% of the patients who had received azathioprine monotherapy (OR 2.54 95% CI 1.00 to 6.78, p value ≤ 0.048). Of note, the week 52 6-TGN levels of the low dose azathioprine and allopurinol group were significantly higher, at a mean of 475 pmol/8 × 108RBC, compared to the azathioprine monotherapy group, with a mean of 303 pmol/8 × 108RBC (p value ≤ 0.001). Further trials including the ongoing DECIDER Study should help clarify the potential role of first line thiopurine-allopurinol combination therapy [76, 79, 85].
In 2018 Friedman et al. [84] reported on 73 patient identified as thiopurine shunters with- ongoing active IBD or steroid-dependence who were randomised to either 50 or 100 mg of allopurinol in combination with low dose azathioprine which was adjusted based on 6-TGN monitoring. 53% (95% CI 42–65) of patients achieved the primary endpoint of steroid-free remission and 81% of patients were able to discontinue steroids. Allopurinol 100 mg was more effective than 50 mg at reducing 6-MMPR levels. The trial also noted that patients who achieved therapeutic 6-TGN had lower average calprotectin levels, reflecting better disease control.
Allopurinol: Mechanism of Action and Safety
Allopurinol has an oral bioavailability of approximately 80% [86]. It has a half-life of one hour, however, the half-life of the active metabolite oxypurinol is much longer at 23 h. Allopurinol is predominately excreted by the kidneys as its active metabolite oxypurinol [86]. Xanthine oxidoreductase, commonly named xanthine oxidase (XO), is the enzyme that is inhibited by allopurinol therapy. XO oxidises xanthines while also directly reducing oxygen to superoxide. However, the mechanism by which allopurinol alters the mechanism of azathioprine is not fully understood. The best explanation to date comes from Blaker et al., published in 2011 [45]. They suggested that when 6-MP enters cells it undergoes four competitive pathways as outlined above. Because allopurinol inhibits the creation of 6-TA (6-thiouric acid) there is greater conversion of 6-MP to 6-TX via aldehyde oxidase. 6-TX then results in direct inhibition of TPMT. This mechanism would explain why patients treated with a combination of thiopurine and allopurinol have significantly decreased 6-MMPR levels. Another possibility is that allopurinol could be inhibiting an enzymic co-factor involved in thiopurine metabolism. Phosphoribosylpyrophosphate (PRPP) is a necessary substrate for several enzymes involved in the synthesis of purine-based ribonucleotides including 6-MMPR. Studies predating metabolite measurements showed that allopurinol causes rapid depletion of erythrocyte PRPP which would therefore inhibits 6-MMPR production [87]. There are also data suggesting that allopurinol itself has anti-inflammatory actions in the treatment of inflammatory bowel disease by the scavenging of intestinal reactive oxygen species [88, 89].
Allopurinol is generally well tolerated with rare but well described side effects that range from mild to potentially life threatening. The most common side effects of allopurinol, occurring in between 1 and 10% of patients treated, are rash, flare of gout, nausea, vomiting and a rise in creatinine [90]. The other rarer reactions that need to be monitored for are drug reaction with eosinophilia and systemic symptoms (DRESS), Steven Johnson’s syndrome, hepatoxicity, cardiac toxicity, bone marrow toxicity and neurotoxicity [90]. Fortunately, DRESS and Stevens-Johnson syndrome from allopurinol are very rare and occur in < 100 per 1,000,000 patient years and < 2 per 1,000,000 person years respectively [91,92,93]. Of note, patients of Asian descent and in particular Han Chinese, associated with their higher incidence of the HLA-B5801 allele, have higher rates of Stevens-Johnson syndrome from allopurinol [92, 94].
Recommendations: How and Where Does This Combination Belong in the Treatment Algorithm
Combination azathioprine and allopurinol occupies an important place in the treatment of IBD. Not only does the combination allow physicians to salvage azathioprine in patients who are shunters or are intolerant, but it also increases the number of clinical scenarios where azathioprine can be used safety.
We suggest the following situations are when combination azathioprine and allopurinol should be used:
-
Patients who demonstrate significant shunting/hypermethylation (6-MMPR:6-TGN ratio > 11)
-
Patients who achieve a target 6-TGN level but develop hepatotoxicity. A trial of switching from azathioprine to mercaptopurine should be tried first if azathioprine was used, but if hepatotoxicity persists allopurinol and reduced dose thiopurine should be commenced
-
Patients who are receiving allopurinol for another indication (most commonly gout), in whom lower thiopurine doses will be required
-
Patients with dose-dependent thiopurine intolerance, independent of metabolite levels, and when metabolite measurements are not available
Once the decision is made to transition a patient to combination azathioprine and allopurinol we suggest the following recommendations:
-
Reduce the dose of azathioprine (same relative reduction for 6-MP) to 25–33% of weight-based target dose (i.e. 0.5–0.7 mg/kg for azathioprine)
-
C–ommence allopurinol at 100 mg daily
-
Monitor FBC, LFT and symptoms every two weeks for the first eight weeks
-
Recheck metabolites (6-MMPR/6-TGN) after four weeks of therapy
-
Continue to monitor bloods every 3 months for the first 12 months
-
If low 6-TGN levels are found on metabolite monitoring first ensure adherence is adequate before proceeding with small (e.g. 25 mg) incremental increases in azathioprine dose
-
If LFT derangement is persistent despite allopurinol co-therapy then consider increasing the allopurinol dose (to 200 or 300 mg) with close monitoring for improvement, or worsening, of LFTs
Conclusions
Combination low dose thiopurine-allopurinol therapy is a practical, safe and effective therapeutic strategy that may allow 15–20% more patients with IBD to tolerate and benefit from thiopurine therapy. Further data from ongoing prospective studies are awaited to confirm whether first line allopurinol-reduced dose thiopurine therapy can be used in all thiopurine-naïve patients. Despite an increasing number of therapies available in the IBD clinic, optimisation of each therapy before switching within or out of class remains good medicine. Although the role of thiopurines in IBD management is likely to reduce with time due to the availability of newer biologics and small molecules, many global jurisdictions will still rely on thiopurines for the foreseeable future. Optimising thiopurines, including with the use of concomitant allopurinol, therefore remains an important option for IBD clinicians, and potentially those managing other immune-mediated inflammatory diseases.
References
Yu H, MacIsaac D, Wong JJ, Sellers ZM, Wren AA, Bensen R et al. Market share and costs of biologic therapies for inflammatory bowel disease in the USA. Aliment Pharmacol Ther. 2018;47:364–370.
Van Der Valk ME, Mangen MJJ, Severs M, Van Der Have M, Dijkstra G, Van Bodegraven AA et al. Evolution of costs of inflammatory bowel disease over two years of follow-up. PLoS One. 2016;11:1–11.
Vasudevan A, Ip F, Liew D, Van Langenberg DR. The Cost-effectiveness of Initial Immunomodulators or Infliximab Using Modern Optimization Strategies for Crohn’s Disease in the Biosimilar Era. Inflamm Bowel Dis. 2020;26:369–379.
Sardesai A, Dignass A, Quon P, Milev S, Cappelleri JC, Kisser A et al. Cost-effectiveness of tofacitinib compared with infliximab, adalimumab, golimumab, vedolizumab and ustekinumab for the treatment of moderate to severe ulcerative colitis in Germany. J Med Econ. 2021;24:279–290.
Park KT, Ehrlich OG, Allen JI, Meadows P, Szigethy EM, Henrichsen K et al. The Cost of Inflammatory Bowel Disease: An Initiative from the Crohn’s & Colitis Foundation. Inflamm Bowel Dis. 2020;26:1–10.
Zeng D, Huang X, Lin S, Lin R, Weng X, Huang P. Cost-effectiveness analysis of genotype screening and therapeutic drug monitoring in patients with inflammatory bowel disease treated with azathioprine therapy: a Chinese healthcare perspective using real-world data. Ann Transl Med. 2021;9(14).
Swann R, Boal A, Squires SI, Lamb C, Clark LL, Lamont S et al. Optimising IBD patient selection for de-escalation of anti-TNF therapy to immunomodulator maintenance. Frontline Gastroenterol. 2020;11:16–21.
Nguyen ALH, Sparrow MP. Evolving Role of Thiopurines in Inflammatory Bowel Disease in the Era of Biologics and New Small Molecules. Dig Dis Sci. 2021;66:3250–3262.
Louis E, Irving P, Beaugerie L. Use of azathioprine in IBD: Modern aspects of an old drug. Gut. 2014 Nov;63:1695–1699.
Singh A, Mahajan R, Kedia S, Dutta AK, Anand A, Bernstein CN et al. Use of thiopurines in inflammatory bowel disease: an update. Intest Res. 2022;20:11–30.
Dharmasiri S, Dewhurst H, Johnson H, Weaver S, McLaughlin S. Low dose thiopurine and allopurinol co-therapy results in significant cost savings at a district general hospital. Frontline Gastroenterol. 2015;6:285–289.
Moreau AC, Paul S, Del Tedesco E, Rinaudo-Gaujous M, Boukhadra N, Genin C et al. Association between 6-thioguanine nucleotides levels and clinical remission in inflammatory disease: A meta-analysis. Inflamm Bowel Dis. 2014;20:464–471.
Mao R, Guo J, Luber R, Chen BL, He Y, Zeng ZR et al. 6-thioguanine nucleotide levels are associated with mucosal healing in patients with Crohn’s disease. Inflamm Bowel Dis. 2018;24:2621–2627.
Pillai N, Dusheiko M, Burnand B, Pittet V. A systematic review of cost-effectiveness studies comparing conventional, biological and surgical interventions for inflammatory bowel disease. PLoS One. 2017;12:1–22.
Putrik P, Ramiro S, Kvien TK, Sokka T, Pavlova M, Uhlig T et al. Inequities in access to biologic and synthetic DMARDs across 46 European countries. Ann Rheum Dis. 2014;73:198–206.
Mahajan R, Singh A, Kedia S, Kaur K, Midha V, Sahu P et al. Maintaining infliximab induced clinical remission with azathioprine and 5-aminosalicylates in acute severe steroid-refractory ulcerative colitis has lower cost and high efficacy (MIRACLE): a multicenter study. Intest Res. 2022;20:64–71.
Sulais E Al, Alameel T. Biosimilars to antitumor necrosis factor agents in inflammatory bowel disease. Biol Targets Ther. 2020;14:1–11.
Gorham P. Cost-Effectiveness Guidelines. Pharmacoeconomics. 1995;8:369–373.
Rogler G, Bernstein CN, Sood A, Goh KL, Yamamoto-Furusho JK, Abbas Z et al. Role of biological therapy for inflammatory bowel disease in developing countries. Gut. 2012 May;61:706–712.
Elion GB. The purine path to chemotherapy. Biosci Rep. 1989;9:509–529.
Skipper HE, Thomson JR, Elion GB, Hitchings GH. Observations on the Anticancer Activity of 6-Mercaptopurine. Cancer Res. 1954;14:294–298.
Bean RH. The treatment of chronic ulcerative colitis with 6-mercaptopurine. Med J Aust. 1962;2:592–593.
Present DH, Korelitz BI, Wisch N, Glass JL, Sachar DB, Pasternack BS. Treatment of Crohn’s Disease with 6-Mercaptopurine. N Engl J Med. 1980;302:981–987.
Broen JCA, van Laar JM. Mycophenolate mofetil, azathioprine and tacrolimus: mechanisms in rheumatology. Nat Rev Rheumatol. 2020;16:167–178.
Coffey JJ, White CA, Lesk AB, Rogers WI, Serpick AA. Effect of Allopurinol on the Pharmacokinetics of 6-Mercaptopurine (NSC 755) in Cancer Patients. Cancer Res. 1972;32:1283–1289.
Klinenberg JR, Goldfinger SE, Seegmiller JE, Bethesda M, Seegmiller JE. The Effectiveness of the Xanthine Oxidase Inhibitor Allopurinol in the Treatment of Gout. Ann Intern Med. 1965;62:639–647.
Chocair P, Ianhez L, Arap S, Sabbaga E, Duley J, Simmonds HA et al. Low-dose allopurinol plus azathioprine/ cyclosporin/prednisolone, a novel immunosuppressive regimen. Lancet. 1993 Jul;342:83–84.
Sparrow MP, Hande SA, Friedman S, Lim WC, Reddy SI, Cao D et al. Allopurinol safely and effectively optimizes tioguanine metabolites in inflammatory bowel disease patients not responding to azathioprine and mercaptopurine. Aliment Pharmacol Ther. 2005 Sep;22:441–446.
Bolia R, Rajanayagam J, Hardikar W. Lower 6-MMP/6-TG Ratio May Be a therapeutic target in pediatric autoimmune hepatitis. J Pediatr Gastroenterol Nutr. 2018;67:695–700.
De Boer YS, Van Gerven NMF, De Boer NKH, Mulder CJJ, Bouma G, Van Nieuwkerk CMJ. Allopurinol safely and effectively optimises thiopurine metabolites in patients with autoimmune hepatitis. Aliment Pharmacol Ther. 2013;37:640–646.
Chande N, Patton PH, Tsoulis DJ, Thomas BS, Macdonald JK. Azathioprine or 6-mercaptopurine for maintenance of remission in Crohn’s disease. Cochrane Database Syst Rev. 2015 Oct;10.
Timmer A, Patton PH, Chande N, Mcdonald JW, Macdonald JK. Azathioprine and 6-mercaptopurine for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2016 May;5.
Torres J, Bonovas S, Doherty G, Kucharzik T, Gisbert JP, Raine T et al. ECCO guidelines on therapeutics in Crohn’s disease: Medical treatment. J Crohn’s Colitis. 2020 Jan;14:4–22.
Raine T, Bonovas S, Burisch J, Kucharzik T, Adamina M, Annese V et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J Crohn’s Colitis. 2021 Oct;16:2–17.
Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;79–80:153–170.
Tidd DM, Paterson ARP. A Biochemical Mechanism for the Delayed Cytotoxic Reaction of 6-Mercaptopurine. Cancer Res. 1974;34:738–746.
Lennard L. TPMT in the treatment of Crohn’s disease with azathioprine. Gut. 2002;51:143–146.
Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–1145.
Marinković G, Kroon J, Hoogenboezem M, Hoeben KA, Ruiter MS, Kurakula K et al. Inhibition of GTPase Rac1 in Endothelium by 6-Mercaptopurine Results in Immunosuppression in Nonimmune Cells: New Target for an Old Drug. J Immunol. 2014;192:4370–4378.
Van Rijssel J, Kroon J, Hoogenboezem M, Van Alphen FPJ, De Jong RJ, Kostadinova E et al. The Rho-guanine nucleotide exchange factor Trio controls leukocyte transendothelial migration by promoting docking structure formation. Mol Biol Cell. 2012;23:2831–2844.
Derijks LJJ, Gilissen LPL, Hooymans PM, Hommes DW. Review article: Thiopurines in inflammatory bowel disease. Aliment Pharmacol Ther. 2006 Sep;24:715–729.
McGovern DPB, Travis SPL, Duley J, Shobowale-Bakre EM, Dalton HR. Azathioprine intolerance in patients with IBD may be imidazole-related and is independent of TPMT activity. Gastroenterology. 2002;122:838–839.
Crawford DJK, Maddocks JL, Jones DN, Szawlowski P. Rational design of novel immunosuppressive drugs: Analogues of azathioprine lacking the 6-mercaptopurine substituent retain or have enhanced immunosuppressive effects. J Med Chem. 1996 Jul;39:2690–2695.
Sauer H, Hantke U, Wilmanns W. Azathioprine lymphocytotoxicity. Potentially lethal damage by its imidazole derivatives. Arzneimittelforschung. 1988 Jun;38(6):820–4.
Blaker PA, Arenas M, Fairbanks L, Irving P, Marinaki AM, Sanderson J. A biochemical mechanism for the role of allopurinol in TMPT inhibition. Gut. 2011 Apr;60:A132–A133.
Dubinsky MC. Azathioprine, 6-mercaptopurine in inflammatory bowel disease: Pharmacology, efficacy, and safety. Clin Gastroenterol Hepatol. 2004 Sep;2:731–743.
Coenen MJH, De Jong DJ, Van Marrewijk CJ, Derijks LJJ, Vermeulen SH, Wong DR et al. Identification of Patients With Variants in TPMT and Dose Reduction Reduces Hematologic Events During Thiopurine Treatment of Inflammatory Bowel Disease. Gastroenterology. 2015;149:907–917.
Weinshilboum R, Sladek S. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–662.
Smith MA, Blaker P, Marinaki AM, Anderson SH, Irving PM, Sanderson JD. Optimising outcome on thiopurines in inflammatory bowel disease by co-prescription of allopurinol. J Crohn’s Colitis. 2012 Oct;6:905–912.
Moriyama T, Nishii R, Perez-Andreu V, Yang W, Klussmann FA, Zhao X et al. NUDT15 Polymorphisms Alter Thiopurine Metabolism and Hematopoietic Toxicity. Nat Genet. 2016;48:367–373.
Dean L. Azathioprine Therapy and TPMT and NUDT15 Genotype. Med Genet Summ [internet]. 2020;(August).
van Gennep S, Konté K, Meijer B, Heymans MW, D’Haens GR, Löwenberg M et al. Systematic review with meta-analysis: risk factors for thiopurine-induced leukopenia in IBD. Aliment Pharmacol Ther. 2019;50:484–506.
Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000 Apr;118:705–713.
Haines ML, Ajlouni Y, Irving PM, Sparrow MP, Rose R, Gearry RB et al. Clinical usefulness of therapeutic drug monitoring of thiopurines in patients with inadequately controlled inflammatory bowel disease. Inflamm Bowel Dis. 2011;17:1301–1307.
Lennard L, Maddocks JL. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983;35:15–18.
Yarur AJ, Gondal B, Hirsch A, Christensen B, Cohen RD, Rubin DT. Higher Thioguanine Nucleotide Metabolite Levels are Associated with Better Long-term Outcomes in Patients with Inflammatory Bowel Diseases. J Clin Gastroenterol. 2018;52:537–544.
Dubinsky MC, Yang H, Hassard PV, Seidman EG, Kam LY, Abreu MT et al. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904–915.
Kiszka-Kanowitz M, Theede K, Thomsen SB, Bjerrum JT, Brynskov J, Gottschalck IB, et al. Low-dose azathioprine and allopurinol versus azathioprine monotherapy in patients with ulcerative colitis (AAUC): An investigator-initiated, open, multicenter, parallel-arm, randomised controlled trial. eClinicalMedicine. 2022;45:1013–32.
Reinshagen M, Schütz E, Armstrong VW, Behrens C, Von Tirpitz C, Stallmach A et al. 6-Thioguanine nucleotide-adapted azathioprine therapy does not lead to higher remission rates than standard therapy in chronic active Crohn disease: Results from a randomized, controlled, open trial. Clin Chem. 2007;53:1306–1314.
Dassopoulos T, Dubinsky MC, Bentsen JL, Martin CF, Galanko JA, Seidman EG et al. Randomised clinical trial: individualised vs weight-based dosing of azathioprine in Crohn’s disease. Aliment Pharmacol Ther. 2014;39:163–175.
Gearry RB, Barclay ML, Burt MJ, Collett JA, Chapman BA. Thiopurine drug adverse effects in a population of New Zealand patients with inflammatory bowel disease. Pharmacoepidemiol Drug Saf. 2004;13:563–567.
Siegel CA, Sands BE. Review article: Practical management of inflammatory bowel disease patients taking immunomodulators. Aliment Pharmacol Ther. 2005;22:1–16.
Jharap B, Seinen ML, De Boer NKH, Van Ginkel JR, Linskens RK, Kneppelhout JC et al. Thiopurine therapy in inflammatory bowel disease patients: Analyses of two 8-year intercept cohorts. Inflamm Bowel Dis. 2010;16:1541–1549.
Hindorf U, Johansson M, Eriksson A, Kvifors E, Almer SHC. Mercaptopurine treatment should be considered in azathioprine intolerant patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29:654–661.
Maruf AA, Wan L, Obrien PJ. Evaluation of azathioprine-induced cytotoxicity in an in vitro rat hepatocyte system. Biomed Res Int. 2014;Jan 1.
Chaparro M, Ordás I, Cabré E, Garcia-Sanchez V, Bastida G, Peñalva M et al. Safety of thiopurine therapy in inflammatory bowel disease: Long-term follow-up study of 3931 patients. Inflamm Bowel Dis. 2013;19:1404–1410.
Costantino G, Furfaro F, Belvedere A, Alibrandi A, Fries W. Thiopurine treatment in inflammatory bowel disease: Response predictors, safety, and withdrawal in follow-up. J Crohn’s Colitis. 2012;6:588–596.
Peyrin-Biroulet L, Khosrotehrani K, Carrat F, Bouvier AM, Chevaux JB, Simon T et al. Increased Risk for Nonmelanoma Skin Cancers in Patients Who Receive Thiopurines for Inflammatory Bowel Disease. Gastroenterology. 2011 Nov;141:1621–1628.
Lopez A, Mounier M, Bouvier AM, Carrat F, Maynadié M, Beaugerie L et al. Increased Risk of Acute Myeloid Leukemias and Myelodysplastic Syndromes in Patients Who Received Thiopurine Treatment for Inflammatory Bowel Disease. Clin Gastroenterol Hepatol. 2014 Aug;12:1324–1329.
Kandiel A, Fraser AG, Korelitz BI, Brensinger C, Lewis JD. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut. 2005 Aug;54:1121–1125.
Khan N, Patel D, Trivedi C, Kavani H, Pernes T, Medvedeva E et al. Incidence of Acute Myeloid Leukemia and Myelodysplastic Syndrome in Patients With Inflammatory Bowel Disease and the Impact of Thiopurines on Their Risk. Am J Gastroenterol. 2021;116:741–747.
Long MD, Martin CF, Pipkin CA, Herfarth HH, Sandler RS, Kappelman MD. Risk of melanoma and nonmelanoma skin cancer among patients with inflammatory bowel disease. Gastroenterology. 2012;143:390–399.
Kobayashi T, Uda A, Udagawa E, Hibi T. Lack of Increased Risk of Lymphoma by Thiopurines or Biologics in Japanese Patients with Inflammatory Bowel Disease: A Large-Scale Administrative Database Analysis. J Crohn’s Colitis. 2020;14:617–623.
Ariyaratnam J, Subramanian V. Association between thiopurine use and nonmelanoma skin cancers in patients with inflammatory bowel disease: A meta-analysis. Am J Gastroenterol. 2014;109:163–169.
Chupin A, Perduca V, Meyer A, Bellanger C, Carbonnel F, Dong C. Systematic review with meta-analysis: comparative risk of lymphoma with anti-tumour necrosis factor agents and/or thiopurines in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2020;52:1289–1297.
van Liere ELSA, Bayoumy AB, Mulder CJJ, Warner B, Hayee B, Mateen BA, et al. Azathioprine with Allopurinol Is a Promising First-Line Therapy for Inflammatory Bowel Diseases. Dig Dis Sci. 2021;Nov(2):1–12.
Thomsen SB, Allin KH, Burisch J, Jensen CB, Hansen S, Gluud LL et al. Outcome of concomitant treatment with thiopurines and allopurinol in patients with inflammatory bowel disease: A nationwide Danish cohort study. United Eur Gastroenterol J. 2020;8:68–76.
Vasudevan A, Beswick L, Friedman AB, Moltzen A, Haridy J, Raghunath A et al. Low-dose thiopurine with allopurinol co-therapy overcomes thiopurine intolerance and allows thiopurine continuation in inflammatory bowel disease. Dig Liver Dis. 2018 Jul;50:682–688.
Pavlidis P, Stamoulos P, Abdulrehman A, Kerr P, Bull C, Duley J et al. Long-term Safety and Efficacy of Low-dose Azathioprine and Allopurinol Cotherapy in Inflammatory Bowel Disease: A Large Observational Study. Inflamm Bowel Dis. 2016 Jun;22:1639–1646.
Hoentjen F, Seinen ML, Hanauer SB, De Boer NKH, Rubin DT, Bouma G et al. Safety and effectiveness of long-term allopurinol-thiopurine maintenance treatment in inflammatory bowel disease. Inflamm Bowel Dis. 2013 Feb;19:363–369.
Ansari A, Elliott T, Baburajan B, Mayhead P, O’Donohue J, Chocair P et al. Long-term outcome of using allopurinol co-therapy as a strategy for overcoming thiopurine hepatotoxicity in treating inflammatory bowel disease. Aliment Pharmacol Ther. 2008 Sep;28:734–741.
Curkovic I, Rentsch KM, Frei P, Fried M, Rogler G, Kullak-Ublick GA et al. Low allopurinol doses are sufficient to optimize azathioprine therapy in inflammatory bowel disease patients with inadequate thiopurine metabolite concentrations. Eur J Clin Pharmacol. 2013;69:1521–1531.
Kiszka-Kanowitz M, Theede K, Mertz-Nielsen A. Randomized clinical trial: a pilot study comparing efficacy of low-dose azathioprine and allopurinol to azathioprine on clinical outcomes in inflammatory bowel disease. Scand J Gastroenterol. 2016 Dec;51:1470–1475.
Friedman AB, Brown SJ, Bampton P, Barclay ML, Chung A, Macrae FA et al. Randomised clinical trial: efficacy, safety and dosage of adjunctive allopurinol in azathioprine/mercaptopurine nonresponders (AAA Study). Aliment Pharmacol Ther. 2018 Apr;47:1092–1102.
Vasudevan A, Con D, Friedman A, Parsons N, De Cruz P, Kashkooli S et al. De novo combination allopurinol–thiopurine versus standard thiopurine in inflammatory bowel disease patients escalating to immunomodulators: A randomized controlled trial (DECIDER study). J Gastroenterol Hepatol. 2020;35:108–157.
Day RO, Graham GG, Hicks M, Mclachlan AJ, Stocker SL, Williams KM. Clinical pharmacokinetics and pharmacodynamics of allopurinol and oxypurinol. Clin Pharmacokinet. 2007;46:623–644.
Fox IH, Wyngaarden JB, Kelley WN. Depletion of Erythrocyte Phosphoribosylpyrophosphate in Man. N Engl J Med. 1970;283:1177–1182.
Järnerot G, Ström M, Danielsson A, Kilander A, Lööf L, Hultcrantz R et al. Allopurinol in addition to 5-aminosalicylic acid based drugs for the maintenance treatment of ulcerative colitis. Aliment Pharmacol Ther. 2000;14:1159–1162.
Salim AS. Allopurinol and dimethyl sulfoxide improve treatment outcomes in smokers with peptic ulcer disease. J Lab Clin Med. 1992 Jun;119:702–709.
Castrejon I, Toledano E, Rosario MP, Loza E, Pérez-Ruiz F, Carmona L. Safety of allopurinol compared with other urate-lowering drugs in patients with gout: a systematic review and meta-analysis. Rheumatol Int. 2015 Jul;35:1127–1137.
Atzori L, Pinna AL, Mantovani L, Ferreli C, Pau M, Mulargia M et al. Cutaneous adverse drug reactions to allopurinol: 10 year observational survey of the dermatology department - Cagliari University (Italy). J Eur Acad Dermatology Venereol. 2012 Nov;26:1424–1430.
Somkrua R, Eickman EE, Saokaew S, Lohitnavy M, Chaiyakunapruk N. Association of HLA-B*5801 allele and allopurinol-induced stevens johnson syndrome and toxic epidermal necrolysis: A systematic review and meta-analysis. BMC Med Genet. 2011 Sep;12:1–10.
Ghislain H, Foong HBB. Allopurinol and the risk of Stevens-Johnson syndrome and toxic epidermal necrolysis. J R Coll Physicians Edinb. 2009;39:144–145.
Hung SL, Chung WH, Liou LB, Chu CC, Lin M, Huang HP et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102:4134–4139.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding was received to assist with the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
AT performed the literature review, drafting and writing of the article under the supervision of MPS. MPS reviewed the manuscript frequently to ensure accuracy and readability.
Corresponding author
Ethics declarations
Conflict of interest
AT has no relevant financial interests to disclose. MPS has received educational grants and research support from Ferring, Orphan, and Gilead, speaker’s fees from Janssen, Abbvie, Ferring, Takeda, Pfizer, and Shire and has served on advisory boards for Janssen, Takeda, Pfizer, Celgene, Abbvie, MSD, and Emerge Health. Non-financial interests: AT and MPS have no relevant non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Turbayne, A.K., Sparrow, M.P. Low-Dose Azathioprine in Combination with Allopurinol: The Past, Present and Future of This Useful Duo. Dig Dis Sci 67, 5382–5391 (2022). https://doi.org/10.1007/s10620-022-07719-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-022-07719-x