Abstract
Background
Preliminary data suggest that treatment optimization can reverse immunogenicity and regain response in patients with IBD and secondary loss of response (SLR) to anti-TNF therapy due to antidrug antibodies. However, data regarding the long-term outcome of these patients are scarce.
Aims
We aimed to investigate drug retention in IBD patients of whom infliximab was optimized to overcome immunogenicity and variables associated with drug retention.
Methods
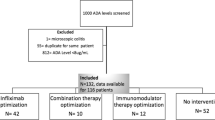
This was a retrospective, multicenter study of consecutive IBD patients with antibodies to infliximab (ATI), based on either proactive or reactive therapeutic drug monitoring, who underwent infliximab optimization (increasing dose, shortening interval, adding an immunomodulator, or combination) to overcome immunogenicity from September 2012 to July 2015; they were followed through December 2015. ATI were analyzed using the drug-tolerant Prometheus homogeneous mobility shift assay. Drug retention was defined as no need for drug discontinuation due to SLR or serious adverse event.
Results
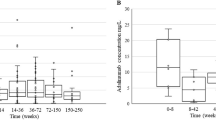
Our cohort consisted of 22 patients (Crohn’s disease, n = 15). At the end of follow-up [median, (IQR): 17.3 (10.5–32.8) months] 77% (15/22) of patients were still on drug. Univariable Cox proportional hazards regression analysis identified first detectable ATI titer as the only variable associated with drug retention (HR: 0.89; 95% CI: 0.82–0.98, p = 0.016). Receiver-operating characteristic analysis identified an ATI titer < 8.8 U/mL associated with drug retention.
Conclusions
In real-life clinical practice, optimization of infliximab therapy can prevent drug discontinuation in approximately 3/4 of patients with ATI, especially in those with low titers. Large prospective studies are needed to confirm these data.




Similar content being viewed by others
References
Miligkos M, Papamichael K, Casteele NV, et al. Efficacy and safety profile of anti-tumor necrosis factor-α versus anti-integrin agents for the treatment of Crohn’s disease: a network meta-analysis of indirect comparisons. Clin Ther. 2016;38:1342–1358.
Papamichael K, Cheifetz AS. Use of anti-TNF drug concentrations to optimise patient management. Frontline Gastroenterol. 2016;7:289–300.
Vande Casteele N, Gils A, Singh S, et al. Antibody response to infliximab and its impact on pharmacokinetics can be transient. Am J Gastroenterol. 2013;108:962–971.
Kopylov U, Mazor Y, Yavzori M, et al. Clinical utility of antihuman lambda chain-based enzyme-linked immunosorbent assay (ELISA) versus double antigen ELISA for the detection of anti-infliximab antibodies. Inflamm Bowel Dis. 2012;18:1628–1633.
Vande Casteele N, Gils A. Pharmacokinetics of anti-TNF monoclonal antibodies in inflammatory bowel disease: adding value to current practice. J Clin Pharmacol. 2015;55:S39–S50.
Vande Casteele N, Khanna R, Levesque BG, et al. The relationship between infliximab concentrations, antibodies to infliximab and disease activity in Crohn’s disease. Gut. 2015;64:1539–1545.
Nanda KS, Cheifetz AS, Moss AC. Impact of antibodies to infliximab on clinical outcomes and serum infliximab concentrations in patients with inflammatory bowel disease (IBD): a meta-analysis. Am J Gastroenterol. 2013;108:40–47.
Yanai H, Lichtenstein L, Assa A, et al. Concentrations of drug and antidrug antibodies are associated with outcome of interventions after loss of response to infliximab or adalimumab. Clin Gastroenterol Hepatol. 2015;13:522–530.
Papamichael K, Karatzas P, Mantzaris GJ. Addition of an immunomodulator as a rescue therapy for loss of response to adalimumab dose escalation in patients with Crohn’s disease. J Crohns Colitis. 2015;9:589–590.
Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis. 2012;18:2026–2033.
Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastroenterol. 2014;109:1250–1256.
Billiet T, Cleynen I, Ballet V, et al. Prognostic factors for long-term infliximab treatment in Crohn’s disease patients: a 20-year single centre experience. Aliment Pharmacol Ther. 2016;44:673–683.
Hindryckx P, Novak G, Vande Casteele N, et al. Incidence, prevention and management of anti-drug antibodies against therapeutic antibodies in inflammatory bowel disease: a practical overview. Drugs. 2017;77:363–377.
Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013;11:444–4447.
Peyrin-Biroulet L, Salleron J, Filippi J, et al. Anti-TNF monotherapy for Crohn’s disease: a 13-year multicentre experience. J Crohns Colitis. 2016;10:516–524.
Strik AS, van den Brink GR, Ponsioen C, et al. Suppression of anti-drug antibodies to infliximab or adalimumab with the addition of an immunomodulator in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2017;45:1128–1134.
Ungar B, Kopylov U, Engel T, et al. Addition of an immunomodulator can reverse antibody formation and loss of response in patients treated with adalimumab. Aliment Pharmacol Ther. 2017;45:276–282.
Papamichael K, Chachu KA, Vajravelu R, et al. Improved long-term outcomes of patients with inflammatory bowel disease receiving proactive compared with reactive monitoring of serum concentrations of infliximab. Clin Gastroenterol Hepatol. 2017. https://doi.org/10.1016/j.cgh.2017.03.031.
Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogeneous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab concentrations in patient serum. J Immunol Methods. 2012;382:177–188.
Dreesen E, Van Stappen T, Ballet V, et al. Anti-infliximab antibody concentrations can guide on treatment intensification in patients with Crohn’s disease who lose clinical response. Aliment Pharmacol Ther. 2017. https://doi.org/10.1111/apt.14452.
Van Stappen T, Vande Casteele N, Van Assche G, et al. Clinical relevance of detecting anti-infliximab antibodies with a drug-tolerant assay: post hoc analysis of the TAXIT trial. Gut. 2017. https://doi.org/10.1136/gutjnl-2016-313071.
Acknowledgments
K.P.: Ruth L. Kirschstein NRSA Institutional Research Training Grant (5T32DK007760-18).
Author information
Authors and Affiliations
Contributions
KP contributed to study concept and design, data acquisition, analysis and interpretation, statistical analysis, and manuscript writing; RV involved in data acquisition and manuscript critical revision, and ASC and MTO contributed to study concept and design, data interpretation, and manuscript critical revision. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
A.S.C received consultancy fees from AbbVie, Janssen, Takeda, Ferring, Samsung, Miraca and Pfizer; M.T.O received consultancy fees from Janssen, AbbVie, UCB, Takeda, Pfizer, Merck, and Lycera and received research grant support from UCB; the remaining authors disclose no conflicts of interest.
Ethical standards
All procedures performed in the study were in accordance with the International Standard of Good Clinical Practice procedures and with the principles of the Declaration of Helsinki (1964) and relevant amendments. The protocol was approved by all institutional review boards and ethics committees at participating sites.
Rights and permissions
About this article
Cite this article
Papamichael, K., Vajravelu, R.K., Osterman, M.T. et al. Long-Term Outcome of Infliximab Optimization for Overcoming Immunogenicity in Patients with Inflammatory Bowel Disease. Dig Dis Sci 63, 761–767 (2018). https://doi.org/10.1007/s10620-018-4917-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-018-4917-7