Abstract
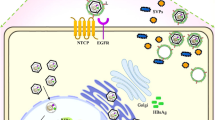
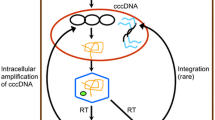
Persistent hepatitis B virus (HBV) infection of hepatocytes is associated with a covalently closed circular DNA (cccDNA) episome. Although serologic hepatitis B surface antigen tests are negative, the presence of cccDNA is obviously increased in HBeAg-positive patients compared with that in HBeAg-negative patients, inactive carriers and patients. Moreover, trace cccDNA levels can also be found in the liver cells of patients with resolved hepatitis B infections. Therefore, clearance of cccDNA in hepatocytes could be an effective cure for HBV. In this review, we summarize the strategies that have been employed to eliminate cccDNA in recent years and discuss the future development of treatments for chronic hepatitis B.

Similar content being viewed by others
References
Gerlich WH. Medical virology of hepatitis B: how it began and where we are now. Virol J. 2013;10:239.
Karayiannis P. Hepatitis B virus: old, new and future approaches to antiviral treatment. J Antimicrob Chemother. 2003;51:761–785.
Xu B, Lin L, Xu G, et al. Long-term lamivudine treatment achieves regression of advanced liver fibrosis/cirrhosis in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:372–378.
Seeger C, Mason WS. Molecular biology of hepatitis B virus infection. Virology. 2015;479–480:672–686.
Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife. 2012;1:e00049.
Kock J, Rosler C, Zhang JJ, Blum HE, Nassal M, Thoma C. Generation of covalently closed circular DNA of hepatitis B viruses via intracellular recycling is regulated in a virus specific manner. PLoS Pathog. 2010;6:e1001082.
Yang HC, Kao JH. Persistence of hepatitis B virus covalently closed circular DNA in hepatocytes: molecular mechanisms and clinical significance. Emerg Microb Infect. 2014;3:e64.
Revill P, Locarnini S. Antiviral strategies to eliminate hepatitis B virus covalently closed circular DNA (cccDNA). Curr Opin Pharmacol. 2016;30:144–150.
Liu F, Campagna M, Qi Y, et al. Alpha-interferon suppresses hepadnavirus transcription by altering epigenetic modification of cccDNA minichromosomes. PLoS Pathog. 2013;9:e1003613.
Li HC, Huang EY, Su PY, et al. Nuclear export and import of human hepatitis B virus capsid protein and particles. PLoS Pathog. 2010;6:e1001162.
Schmitz A, Schwarz A, Foss M, et al. Nucleoporin 153 arrests the nuclear import of hepatitis B virus capsids in the nuclear basket. PLoS Pathog. 2010;6:e1000741.
Zlotnick A, Venkatakrishnan B, Tan Z, Lewellyn E, Turner W, Francis S. Core protein: a pleiotropic keystone in the HBV lifecycle. Antiviral Res. 2015;121:82–93.
Lucifora J, Protzer U. Attacking hepatitis B virus cccDNA–The holy grail to hepatitis B cure. J Hepatol. 2016;64:S41.
Schreiner S, Nassal M. A role for the host DNA damage response in hepatitis B virus cccDNA formation—and beyond? Viruses. 2017;9:125.
Baumert TF, Verrier ER, Nassal M, Chung RT, Zeisel MB. Host-targeting agents for treatment of hepatitis B virus infection. Curr Opin Virol. 2015;14:41.
Königer C, Wingert I, Marsmann M, Rösler C, Beck J, Nassal M. Involvement of the host DNA-repair enzyme TDP2 in formation of the covalently closed circular DNA persistence reservoir of hepatitis B viruses. Proc Natl Acad Sci USA. 2014;111:E4244.
Bock CT, Schranz P, Schroder CH, Zentgraf H. Hepatitis B virus genome is organized into nucleosomes in the nucleus of the infected cell. Virus Genes. 1994;8:215–229.
Pollicino T, Belloni L, Raffa G, et al. Hepatitis B virus replication is regulated by the acetylation status of hepatitis B virus cccDNA-bound H3 and H4 histones. Gastroenterology. 2006;130:823–837.
Belloni L, Pollicino T, Nicola FD, et al. Nuclear HBx binds the HBV minichromosome and modifies the epigenetic regulation of cccDNA function. J Neurochem. 2002;83:19975–19979.
Belloni L, Allweiss L, Guerrieri F, et al. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Investig. 2012;122:529–537.
Lutgehetmann M, Volz T, Köpke A, et al. In vivo proliferation of hepadnavirus-infected hepatocytes induces loss of covalently closed circular DNA in mice. Hepatology. 2010;52:16–24.
Guo JT, Pryce M, Wang X, Barrasa MI, Hu J, Seeger C. Conditional replication of duck hepatitis B virus in hepatoma cells. J Virol. 2003;77:1885.
Summers J, Jilbert AR, Yang W, et al. Hepatocyte turnover during resolution of a transient hepadnaviral infection. Proc Natl Acad Sci USA. 2003;100:11652.
Reaichemiller GY, Thorpe M, Low HC, et al. Duck hepatitis B virus covalently closed circular DNA appears to survive hepatocyte mitosis in the growing liver. Virology. 2013;446:357–364.
Allweiss L, Volz T, Giersch K, et al. Proliferation of Hepatitis B virus infected human hepatocytes induces suppression of viral replication and rapid cccDNA decrease in humanized mice. J Hepatol. 2013;58:S56–S57.
Werle-Lapostolle B, Bowden S, Locarnini S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126:1750–1758.
Blanpain C, Knoop C, Delforge ML, et al. Reactivation of hepatitis B after transplantation in patients with pre-existing anti-hepatitis B surface antigen antibodies: report on three cases and review of the literature. Transplantation. 1998;66:883–886.
Palmore TN, Shah NL, Loomba R, et al. Reactivation of hepatitis B with reappearance of hepatitis B surface antigen after chemotherapy and immunosuppression. Clin Gastroenterol Hepatol. 2009;7:1130–1137.
Hung HH, Su CW, Wu JC, Lee SD. Reactivation of hepatitis B virus after transarterial chemo-embolization for hepatocellular carcinoma in one patient with negative hepatitis B surface antigen. J Hepatol. 2010;52:463–465.
Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology. 2014;60:2099–2108.
Nassal M. HBV cccDNA: viral persistence reservoir and key obstacle for a cure of chronic hepatitis B. Gut. 2015;64:1972.
Lanford RE, Guerra B, Chavez D, et al. GS-9620, an oral agonist of Toll-like receptor-7, induces prolonged suppression of hepatitis B virus in chronically infected chimpanzees. Gastroenterology. 2013;144:1508–1517.
Menne S, Tumas DB, Liu KH, et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J Hepatol. 2015;62:1237–1245.
Xia Y, Protzer U. Control of hepatitis B virus by cytokines. Viruses. 2017;9:18.
Schluep T, Lickliter J, Hamilton J, et al. Safety, tolerability, and pharmacokinetics of ARC-520 injection, an RNA interference-based therapeutic for the treatment of chronic hepatitis B virus infection, in healthy volunteers. Clin Pharmacol Drug Dev. 2017;6(4):350–362.
Lok AS, Pan CQ, Han SH, et al. Randomized phase II study of GS-4774 as a therapeutic vaccine in virally suppressed patients with chronic hepatitis B. J Hepatol. 2016;65:509–516.
Mahtab MA, Akbar F, Uddin H, et al. A phase III clinical trial with a nasal vaccine containing both HBsAg and HBcAg in patients with chronic hepatitis B. J Hepatol. 2013;58:S309–S309.
Betancourt AA, Delgado CA, Estevez ZC, et al. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int J Infect Dis. 2007;11:394–401.
Al-Mahtab M, Akbar SM, Aguilar JC, Uddin MH, Khan MS, Rahman S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol Int. 2013;7:981–989.
Xu DZ, Wang XY, Shen XL, et al. Results of a phase III clinical trial with an HBsAg-HBIG immunogenic complex therapeutic vaccine for chronic hepatitis B patients: experiences and findings. J Hepatol. 2013;59:450–456.
Lucifora J, Xia Y, Reisinger F, et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science. 2014;344:1221.
Xia Y, Stadler D, Lucifora J, et al. Interferon-γ and tumor necrosis factor-α produced by t cells reduce the HBV persistence form, cccDNA, without cytolysis. Gastroenterology. 2016;150:194–205.
Bockmann JH, Xia Y, Stadler D, Protzer U. Type III interferons induce cccDNA degradation similar to type I interferons in HBV-infected HepaRG cells. Zeitschrift Für Gastroenterologie. 2015. https://doi.org/10.1055/s-0034-1397258.
Qiao Y, Han X, Guan G, et al. TGF-beta triggers HBV cccDNA degradation through AID-dependent deamination. FEBS Lett. 2016;590:419–427.
Fidaa B, Olivier F, Philippe G. Interleukin 6 inhibits HBV entry through NTCP down regulation. Virology. 2015;481:34–42.
Hösel M, Quasdorff M, Wiegmann K, et al. Not interferon, but interleukin-6 controls early gene expression in hepatitis B virus infection. Hepatology. 2009;50:1773.
Palumbo GA, Scisciani C, Pediconi N, et al. IL6 inhibits HBV transcription by targeting the epigenetic control of the nuclear cccDNA minichromosome. PLoS ONE. 2015;10:e0142599.
Weber ND, Stone D, Sedlak RH, et al. AAV-mediated delivery of zinc finger nucleases targeting hepatitis B virus inhibits active replication. PLoS ONE. 2014;9:e97579.
Bloom K, Ely A, Mussolino C, Cathomen T, Arbuthnot P. Inactivation of hepatitis B virus replication in cultured cells and in vivo with engineered transcription activator-like effector nucleases. Mol Ther. 2013;21:1889–1897.
Chen J, Zhang W, Lin J, et al. An efficient antiviral strategy for targeting hepatitis B virus genome using transcription activator-like effector nucleases. Mol Ther. 2014;22:303–311.
Dreyer T, Nicholson S, Ely A, Arbuthnot P, Bloom K. Improved antiviral efficacy using TALEN-mediated homology directed recombination to introduce artificial primary miRNAs into DNA of hepatitis B virus. Biochem Biophys Res Commun. 2016;478:1563–1568.
Qi Y, Gao Z, Xu G, et al. DNA polymerase kappa is a key cellular factor for the formation of covalently closed circular DNA of hepatitis B virus. PLoS Pathog. 2016;12:e1005893.
Zhu W, Xie K, Xu Y, et al. CRISPR/Cas9 produces anti-hepatitis B virus effect in hepatoma cells and transgenic mouse. Virus Res. 2016;217:125–132.
Lin SR, Yang HC, Kuo YT, et al. The CRISPR/Cas9 system facilitates clearance of the intrahepatic HBV templates in vivo. Mol Ther Nucl Acids. 2014;3:e186.
Dong C, Qu L, Wang H, Wei L, Dong Y, Xiong S. Targeting hepatitis B virus cccDNA by CRISPR/Cas9 nuclease efficiently inhibits viral replication. Antiviral Res. 2015;118:110–117.
Moore MD, McGarvey MJ, Russell RA, Cullen BR, McClure MO. Stable inhibition of hepatitis B virus proteins by small interfering RNA expressed from viral vectors. J Gene Med. 2005;7:918–925.
Uprichard SL, Boyd B, Althage A, Chisari FV. Clearance of hepatitis B virus from the liver of transgenic mice by short hairpin RNAs. Proc Natl Acad Sci USA. 2005;102:773–778.
Peng J, Zhao Y, Mai J, et al. Inhibition of hepatitis B virus replication by various RNAi constructs and their pharmacodynamic properties. J Gen Virol. 2005;86:3227–3234.
Morrissey DV, Lockridge JA, Shaw L, et al. Potent and persistent in vivo anti-HBV activity of chemically modified siRNAs. Nat Biotechnol. 2005;23:1002–1007.
Li G, Fu L, Jiang J, Ping Y, Huang Y, Wang Y. siRNA combinations mediate greater suppression of hepatitis B virus replication in mice. Cell Biochem Biophys. 2014;69:641–647.
Wooddell CI, Rozema DB, Hossbach M, et al. Hepatocyte-targeted RNAi therapeutics for the treatment of chronic hepatitis B virus infection. Mol Ther. 2013;21:973–985.
Thongthae N, Payungporn S, Poovorawan Y, T-Thienprasert NP. A rational study for identification of highly effective siRNAs against hepatitis B virus. Exp Mol Pathol. 2014;97:120–127.
Sebestyen MG, Wong SC, Trubetskoy V, Lewis DL, Wooddell CI. Targeted in vivo delivery of siRNA and an endosome-releasing agent to hepatocytes. Methods Mol Biol. 2015;1218:163–186.
Durantel D, Zoulim F. New antiviral targets for innovative treatment concepts for hepatitis B virus and hepatitis delta virus. J Hepatol. 2016;64:S117–131.
Gish RG, Yuen MF, Chan HL, et al. Synthetic RNAi triggers and their use in chronic hepatitis B therapies with curative intent. Antiviral Res. 2015;121:97–108.
Kim JW, Lee SH, Park YS, et al. Replicative activity of hepatitis B virus is negatively associated with methylation of covalently closed circular DNA in advanced hepatitis B virus infection. Intervirology. 2011;54:316–325.
Vivekanandan P, Thomas D, Torbenson M. Hepatitis B viral DNA is methylated in liver tissues. J Viral Hepat. 2008;15:103–107.
Zhang Y, Li C, Zhang Y, et al. Comparative analysis of CpG islands among HBV genotypes. PLoS ONE. 2013;8:e56711.
Zhang Y, Mao R, Yan R, et al. Transcription of hepatitis B virus covalently closed circular DNA is regulated by CpG methylation during chronic infection. PLoS ONE. 2014;9:e110442.
Wang H, Liu K, Fang BA, et al. Identification of acetyltransferase genes (HAT1 and KAT8) regulating HBV replication by RNAi screening. Cell Biosci. 2015;5:66.
Lai CL, Wong D, Ip P, et al. Reduction of covalently closed circular DNA with long-term nucleos(t)ide analogue treatment in chronic hepatitis B. J Hepatol. 2017;66(2):275–281.
Cai D, Mills C, Yu W, et al. Identification of disubstituted sulfonamide compounds as specific inhibitors of hepatitis B virus covalently closed circular DNA formation. Antimicrob Agents Chemother. 2012;56:4277–4288.
Volz T, Allweiss L, Ben MM, et al. The entry inhibitor Myrcludex-B efficiently blocks intrahepatic virus spreading in humanized mice previously infected with hepatitis B virus. J Hepatol. 2013;58:861.
Urban S, Bartenschlager R, Kubitz R, Zoulim F. Strategies to inhibit entry of HBV and HDV into hepatocytes. Gastroenterology. 2014;147:48–64.
Author information
Authors and Affiliations
Contributions
JD and JY compiled the complete draft and modification of the manuscript. XQ and YL provided critiques on the work and then finalized the article prior to submission. The corresponding author, MZ, was responsible for communication with the editor.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Dong, J., Ying, J., Qiu, X. et al. Advanced Strategies for Eliminating the cccDNA of HBV. Dig Dis Sci 63, 7–15 (2018). https://doi.org/10.1007/s10620-017-4842-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-017-4842-1