Abstract
Background
Celiac serology is crucial for the diagnosis of celiac disease in children. The American guideline for celiac disease in children suggested that positive serology should be followed by confirmatory intestinal histology. The relationship between high tissue transglutaminase titers and celiac disease in children has not been well investigated in children from North America.
Aims
In the present study, we investigated whether different tissue transglutaminase titers in symptomatic children could predict celiac disease without the confirmation of intestinal histology.
Methods
Data from biopsy confirmed celiac children were collected from four different clinics in North America. Clinical, serological, histological, and follow-up data were collected. The accuracy rates of various tissue transglutaminase titers to predict celiac disease in children were calculated.
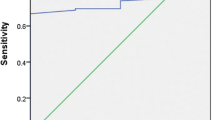
Results
The data from 240 children were calculated. The sensitivity, specificity, positive predictive value, negative predictive value, and accuracy rate of tissue transglutaminase titers at ≥10× upper limit of normal were 75.4, 48.8, 87.7, 29.0, and 70.8 %, respectively. Similar data were noted in the other tissue transglutaminase titers (≥3× upper limit of normal, >100 U/ml, or >100 U/ml and >10× upper limit of normal).
Conclusions
The positive predictive value of tissue transglutaminase titers at ≥3× upper limit of normal or higher was too low to predict celiac disease in children. Our data suggested that in routine clinical practice, high titers of tissue transglutaminase are not sufficient to diagnose celiac disease in North American children without intestinal biopsies.
Similar content being viewed by others
References
Fasano A, Berti I, Gerarduzzi T, et al. Prevalence of celiac disease in at-risk and non-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003;163:286–292.
Fassano J, Araya M, Bhatnagar S, et al. Federation of international societies of Pediatric Gastroenterology Hepatology and Nutrition consensus report on celiac disease. Pediat Gastroenterol Nutr. 2008;47:214–219.
Revised criteria of diagnosis of celiac disease. Report of working group of European Society of Pediatric Gastroenterology and Nutrition. Arch Dis Child. 1990;65:909–911.
Mubarak A, Wolters VM, Gmelig-Meyling FH, Ten Kate FJ, Houwen RH. Tissue transglutaminase levels above 100 U/ml and celiac disease: a prospective study. World J World J Gastroenterol. 2012;18:4399–4403.
Alessio MG, Tonutti E, Brusca I, et al. Correlation between IgA tissue transglutaminase antibody ratio and histological finding in celiac disease. J Pediatr Gastroenterol Nutr. 2012;55:44–49.
Hill PG, Holmes GK. Celiac disease: a biopsy is not always necessary for diagnosis. Aliment Pharmacol Ther. 2008;27:572–577.
Donaldson MR, Firth SD, Wimpee H, et al. Duodenal histology correlates with tissue transglutaminase and endomysial antibody levels in pediatric celiac disease. Clin Gastroenterol Hepatol. 2007;5:567–573.
Webb C, Norström F, Myléus A, et al. Celiac disease can be predicted by high levels of anti-tissue transglutaminase antibodies in population-based screening. J Pediatr Gastroenterol Nutr. 2015;60:787–791.
Husby S, Koletzko S, Korponay-Szabó IR, et al. European society for pediatric gastroenterology, hepatology, and nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012;54:136–160.
Hasegawa K, Ahn J, Brown MA, et al. Underuse of guideline recommended long-term asthma management in children hospitalized to the intensive care unit: a multicenter observational study. Ann Allergy Asthma Immunol. 2015;115:10-6.e1.
Silvester JA, Rashid M. Long-term management of patients with celiac disease: current practices of gastroenterologists in Canada. Can J Gastroenterol. 2010;24:499–509.
Rashid M. Diagnosing celiac disease with a positive serological test and without intestinal biopsy. Pediatrics. 2005;116:1054–1055.
Hill ID, Horvath K. Nonbiopsy diagnosis of celiac disease: are we nearly there yet?. J Pediatr Gastroenterol Nutr. 2012;54:310–311.
Donate E, Ramos JM, Sanchez-Valverde F, et al. Ribes-Koninckx C on behalf of the SEGHNP working group on celiac disease. J Pediatr Gastroenterol Nutr. 2016;62:284–291.
Green PH, Jabri B. Celiac disease. Ann Rev Med. 2006;57:207–221.
Barker CC, Mitton C, Jevon G, Mock T. Can tissue transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115:1341–1346.
Collin P, Kaukinen K, Vogelsang H, et al. Antiendomysial and antihuman recombinant tissue transglutaminase antibodies in the diagnosis of coeliac disease: a biopsy-proven European multicentre study. Eur J Gastroenterol Hepatol. 2005;17:85–91.
Donaldson MR, Book LS, Leiferman KM, Zone JJ, Neuhausen SL. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric. J Clin Gastroenterol. 2008;42:256–260
Gidrewicz D, Potter K, Trevenen CL, Lyon M, Butzner JD. Evaluation of the ESPGHAN celiac guidelines in a North American pediatric population. Am J Gastroenterol. 2015;110:760–767.
Ediger TR, Hill ID. Celiac disease. Pediatr Rev. 2014;35:409–415.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This study was self-funded and the authors have no financial disclosures to make regarding this body of work.
Rights and permissions
About this article
Cite this article
Elitsur, Y., Sigman, T., Watkins, R. et al. Tissue Transglutaminase Levels Are Not Sufficient to Diagnose Celiac Disease in North American Practices Without Intestinal Biopsies. Dig Dis Sci 62, 175–179 (2017). https://doi.org/10.1007/s10620-016-4354-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4354-4