Abstract
Background
Mesenchymal stromal cells (MSCs) have been used in the treatment of Crohn’s disease (CD) because of the immunomodulatory ability.
Aim
The aim of this study was to investigate the therapeutic effect of adipose-derived MSCs (AD-MSCs) and to compare the therapeutic effect of AD-MSCs with that of bone marrow MSCs (BM-MSCs) in a murine model of CD.
Methods
Murine colitis model of CD was created by trinitrobenzene sulfonic acid (TNBS). Twelve hours after treatment with TNBS, the mouse model was injected with MSCs intraperitoneally. Real-time polymerase chain reaction and immunohistochemistry staining were used to measure the expression levels of inflammatory cytokines in colonic tissues to investigate the therapeutic effect of AD-MSCs. The ten-day survival was recorded after infusion of MSCs.
Results
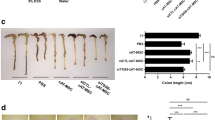
Intraperitoneal injection of MSCs alleviated the clinical and histopathologic severity of intestinal inflammation, and improved the survival of the TNBS-induced mouse model of CD. AD-MSCs could effectively increase the expression of interleukin-10 and reduce the secretion of pro-inflammatory cytokines including tumor necrosis factor-α, interleukin-12, and vascular endothelial growth factor. The mucosal injury was repaired by AD-MSCs. These effects were comparable between AD-MSCs and BM-MSCs.
Conclusions
The therapeutic effect appears similar between AD-MSCs and BM-MSCs in treating CD. AD-MSCs may be a potential alternative of cell-based therapy for CD.






Similar content being viewed by others
References
Crohn BB, Ginzburg L, Oppenheimer GD. Regional ileitis: a pathologic and clinical entity. 1932. Mt Sinai J Med. 2000;67:263–268.
Shaffer VO, Wexner SD. Surgical management of Crohn’s disease. Langenbecks Arch Surg. 2013;398:13–27.
Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990;99:956–963.
Fornaro R, Frascio M, Stabilini C, et al. Crohn’s disease surgery: problems of postoperative recurrence. Chir Ital. 2008;60:761–781.
Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–533.
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347:417–429.
Taupin P. OTI-010 osiris therapeutics/JCR pharmaceuticals. Curr Opin Investig Drugs. 2006;7:473–481.
Ciccocioppo R, Bernardo ME, Sgarella A, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60:788–798.
Duijvestein M, Vos AC, Roelofs H, et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: results of a phase I study. Gut. 2010;59:1662–1669.
Galipeau J. The mesenchymal stromal cells dilemma–does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy. 2013;15:2–8.
Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–228.
Chamberlain G, Fox J, Ashton B, Middleton J. Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells. 2007;25:2739–2749.
Puissant B, Barreau C, Bourin P, et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol. 2005;129:118–129.
Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24:2582–2591.
Bassi G, Pacelli L, Carusone R, Zanoncello J, Krampera M. Adipose-derived stromal cells (ASCs). Transfus Apher Sci. 2012;47:193–198.
Lee RH, Kim B, Choi I, et al. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324.
De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109.
Garcia-Olmo D, Garcia-Arranz M, Garcia LG, et al. Autologous stem cell transplantation for treatment of rectovaginal fistula in perianal Crohn’s disease: a new cell-based therapy. Int J Colorectal Dis. 2003;18:451–454.
Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48:1416–1423.
Garcia-Olmo D, Herreros D, Pascual I, et al. Expanded adipose-derived stem cells for the treatment of complex perianal fistula: a phase II clinical trial. Dis Colon Rectum. 2009;52:79–86.
Soleimani M, Nadri S. A protocol for isolation and culture of mesenchymal stem cells from mouse bone marrow. Nat Protoc. 2009;4:102–106.
Sung JH, Yang HM, Park JB, et al. Isolation and characterization of mouse mesenchymal stem cells. Transplant Proc. 2008;40:2649–2654.
Taha MF, Hedayati V. Isolation, identification and multipotential differentiation of mouse adipose tissue-derived stem cells. Tissue Cell. 2010;42:211–216.
Horwitz EM, Le Blanc K, Dominici M, et al. Clarification of the nomenclature for MSC: the International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393–395.
Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301.
Penick KJ, Solchaga LA, Welter JF. High-throughput aggregate culture system to assess the chondrogenic potential of mesenchymal stem cells. Biotechniques. 2005;39:687–691.
Duijvestein M, Wildenberg ME, Welling MM, et al. Pretreatment with interferon-gamma enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549–1558.
Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147.
Da SML, Sand TT, Harman RJ, Lennon DP, Caplan AI. MSC frequency correlates with blood vessel density in equine adipose tissue. Tissue Eng Part A. 2009;15:221–229.
Hoogduijn MJ, Betjes MG, Baan CC. Mesenchymal stromal cells for organ transplantation: different sources and unique characteristics? Curr Opin Organ Transplant. 2014;19:41–46.
Lee SY, Kim W, Lim C, Chung SG. Treatment of lateral epicondylosis by using allogeneic adipose-derived mesenchymal stem cells: a pilot study. Stem Cells. 2015;33:2995–3005.
Cho YB, Park KJ, Yoon SN, et al. Long-term results of adipose-derived stem cell therapy for the treatment of Crohn’s fistula. Stem Cells Transl Med. 2015;4:532–537.
Toyserkani NM, Christensen ML, Sheikh SP, Sorensen JA. Adipose-derived stem cells: new treatment for wound healing? Ann Plast Surg. 2015;75:117–123.
Danisovic L, Varga I, Polak S, et al. Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen Physiol Biophys. 2009;28:56–62.
Elman JS, Li M, Wang F, Gimble JM, Parekkadan B. A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm (Lond). 2014;11:1.
Ertas G, Ural E, Ural D, et al. Comparative analysis of apoptotic resistance of mesenchymal stem cells isolated from human bone marrow and adipose tissue. Sci World J. 2012;2012:105698.
Rasmussen JG, Frobert O, Holst-Hansen C, et al. Comparison of human adipose-derived stem cells and bone marrow-derived stem cells in a myocardial infarction model. Cell Transplant. 2014;23:195–206.
Huang JI, Kazmi N, Durbhakula MM, Hering TM, Yoo JU, Johnstone B. Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res. 2005;23:1383–1389.
Napoli C, Williams-Ignarro S, de Nigris F, et al. Beneficial effects of concurrent autologous bone marrow cell therapy and metabolic intervention in ischemia-induced angiogenesis in the mouse hindlimb. Proc Natl Acad Sci USA. 2005;102:17202–17206.
Rodrigo SF, van Ramshorst J, Hoogslag GE, et al. Intramyocardial injection of autologous bone marrow-derived ex vivo expanded mesenchymal stem cells in acute myocardial infarction patients is feasible and safe up to 5 years of follow-up. J Cardiovasc Transl Res. 2013;6:816–825.
Iyer SS, Rojas M. Anti-inflammatory effects of mesenchymal stem cells: novel concept for future therapies. Exp Opin Biol Ther. 2008;8:569–581.
Gonzalez MA, Gonzalez-Rey E, Rico L, Buscher D, Delgado M. Adipose-derived mesenchymal stem cells alleviate experimental colitis by inhibiting inflammatory and autoimmune responses. Gastroenterology. 2009;136:978–989.
Gonzalez-Rey E, Anderson P, Gonzalez MA, Rico L, Buscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929–939.
Nemeth K, Leelahavanichkul A, Yuen PS, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49.
Bernardo ME, Locatelli F, Fibbe WE. Mesenchymal stromal cells. Ann N Y Acad Sci. 2009;1176:101–117.
Hass R, Kasper C, Bohm S, Jacobs R. Different populations and sources of human mesenchymal stem cells (MSC): a comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal. 2011;9:12.
Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24:150–154.
Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42.
Acknowledgments
This study is supported by Guangdong Provincial Scientific Technology Foundation (#B2012149), National Natural Science Foundation of China (#81200332, #81300367, and #81570596), Pearl River S&T Nova Program of Guangzhou (#2014J2200040), Science and Technology Planning Project of Guangdong Province (#2015B020229001), and the Fundamental Research Funds for the Central Universities (#15ykjc06e).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Additional information
Minghao Xie and Huabo Qin have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Xie, M., Qin, H., Luo, Q. et al. Comparison of Adipose-Derived and Bone Marrow Mesenchymal Stromal Cells in a Murine Model of Crohn’s Disease. Dig Dis Sci 62, 115–123 (2017). https://doi.org/10.1007/s10620-016-4166-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-016-4166-6