Abstract
Background
Cell division cycle 5-like (Cdc5L), as a pre-mRNA splicing factor, is a regulator of mitotic progression. Previous study found that deletion of endogenous Cdc5L decreases the cell viability via dramatic mitotic arrest, while the role of Cdc5L in cancer biology remains under debate.
Aims
To investigate the involvement of Cdc5L in the progression of hepatocellular carcinoma (HCC).
Methods
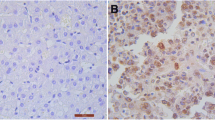
In this study, the expression of Cdc5L was evaluated by Western blot in 8 paired fresh HCC tissues and immunohistochemistry on 116 paraffin-embedded slices. We treated HCC cells by nocodazole to analyze the role of Cdc5L in mitotic progress. To determine whether Cdc5L could regulate the proliferation of HCC cells, we increased endogenous Cdc5L and analyzed the proliferation of HCC cells using Western blot, CCK8, flow cytometry assays, and colony formation analyses. Furthermore, Cdc5L-siRNA oligos were used to confirm that Cdc5L plays an essential role in HCC development.
Results
Cdc5L was highly expressed in HCC and significantly associated with multiple clinicopathological factors, including AJCC stage, tumor size, and Ki-67. Besides, univariate and multivariate survival analyses demonstrated that high Cdc5L expression was an independent prognostic factor for HCC patients’ poor survival. Overexpression of Cdc5L favors cell cycle progress of HCC cells, while downregulation of Cdc5L results in cell cycle arrest at G2/M phase and reduced cell proliferation of HCC cells.
Conclusions
Our findings suggested that Cdc5L could play an important role in the tumorigenesis of HCC and thus be a potential therapeutical target to prevent HCC progression.







Similar content being viewed by others
References
He L, Zhou X, Qu C, et al. Musashi2 predicts poor prognosis and invasion in hepatocellular carcinoma by driving epithelial-mesenchymal transition. J Cell Mol Med. 2014;18:49–58.
Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–S16.
Coleman WB. Mechanisms of human hepatocarcinogenesis. Curr Mol Med. 2003;3:573–588.
Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9:153–166.
Burns CG, Ohi R, Krainer AR, Gould KL. Evidence that Myb-related CDC5 proteins are required for pre-mRNA splicing. Proc Natl Acad Sci USA. 1999;96:13789–13794.
Ajuh P, Kuster B, Panov K, Zomerdijk JC, Mann M, Lamond AI. Functional analysis of the human CDC5L complex and identification of its components by mass spectrometry. EMBO J. 2000;19:6569–6581.
Ajuh P, Sleeman J, Chusainow J, Lamond AI. A direct interaction between the carboxyl-terminal region of CDC5L and the WD40 domain of PLRG1 is essential for pre-mRNA splicing. J Biol Chem. 2001;276:42370–42381.
Ajuh P, Lamond AI. Identification of peptide inhibitors of pre-mRNA splicing derived from the essential interaction domains of CDC5L and PLRG1. Nucleic Acids Res. 2003;31:6104–6116.
Grote M, Wolf E, Will CL, et al. Molecular architecture of the human Prp19/CDC5L complex. Mol Cell Biol. 2010;30:2105–2119.
Wan L, Huang J. The PSO4 protein complex associates with replication protein A (RPA) and modulates the activation of ataxia telangiectasia-mutated and Rad3-related (ATR). J Biol Chem. 2014;289:6619–6626.
Mu R, Wang YB, Wu M, et al. Depletion of pre-mRNA splicing factor Cdc5L inhibits mitotic progression and triggers mitotic catastrophe. Cell Death Dis. 2014;5:e1151.
Huang GJ, Zhang ZQ, Yao LH, Chen AJ, Xu CX. [Screening of new binding partners of CIKS with yeast two-hybrid system] Sheng wu gong cheng xue bao. Chin J Biotechnol. 2003;19:190–194.
Boudrez A, Beullens M, Groenen P, et al. NIPP1-mediated interaction of protein phosphatase-1 with CDC5L, a regulator of pre-mRNA splicing and mitotic entry. J Biol Chem. 2000;275:25411–25417.
Schwartz GK, Shah MA. Targeting the cell cycle: a new approach to cancer therapy. J Clin Oncol. 2005;23:9408–9421.
Asghar U, Witkiewicz AK, Turner NC, Knudsen ES. The history and future of targeting cyclin-dependent kinases in cancer therapy. Nat Rev Drug Discov. 2015;14:130–146.
Sadikovic B, Thorner P, Chilton-Macneill S, et al. Expression analysis of genes associated with human osteosarcoma tumors shows correlation of RUNX2 overexpression with poor response to chemotherapy. BMC Cancer. 2010;10:202.
Martin JW, Chilton-MacNeill S, Koti M, van Wijnen AJ, Squire JA, Zielenska M. Digital expression profiling identifies RUNX2, CDC5L, MDM2, RECQL4, and CDK4 as potential predictive biomarkers for neo-adjuvant chemotherapy response in paediatric osteosarcoma. PloS ONE. 2014;9:e95843.
Kreppel M, Amir Manawi NN, Scheer M, et al. Prognostic quality of the Union Internationale Contre le Cancer/American Joint Committee on Cancer TNM classification, 7th edition, for cancer of the maxillary sinus. Head Neck. 2015;37:400–406.
Wan C, Hou S, Ni R, et al. MIF4G domain containing protein regulates cell cycle and hepatic carcinogenesis by antagonizing CDK2-dependent p27 stability. Oncogene. 2015;34:237–245.
Wan C, Liu J, Nie X, et al. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PloS ONE. 2014;9:e89811.
Poli A, Ramazzotti G, Matteucci A, et al. A novel DAG-dependent mechanism links PKCa and Cyclin B1 regulating cell cycle progression. Oncotarget. 2014;5:11526–11540.
Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543.
Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234.
Moiseeva TN, Bottrill A, Melino G, Barlev NA. DNA damage-induced ubiquitylation of proteasome controls its proteolytic activity. Oncotarget. 2013;4:1338–1348.
Clute P, Pines J. Temporal and spatial control of cyclin B1 destruction in metaphase. Nat Cell Biol. 1999;1:82–87.
Sun H, Gao Y, Lu K, et al. Overexpression of Klotho suppresses liver cancer progression and induces cell apoptosis by negatively regulating wnt/β-catenin signaling pathway. World J Surg Oncol. 2015;13:307.
Goh KL, Razlan H, Hartono JL, et al. Liver cancer in Malaysia: epidemiology and clinical presentation in a multiracial Asian population. J Dig Dis. 2015;16:152–158.
Liu WT, Jing YY, Yu GF, et al. Toll like receptor 4 facilitates invasion and migration as a cancer stem cell marker in hepatocellular carcinoma. Cancer Lett. 2015;358:136–143.
Chen RC, Yi PP, Zhou RR, et al. The role of HMGB1-RAGE axis in migration and invasion of hepatocellular carcinoma cell lines. Mol Cell Biochem. 2014;390:271–280.
Rao CV, Kurkjian CD, Yamada HY. Mitosis-targeting natural products for cancer prevention and therapy. Curr Drug Target. 2012;13:1820–1830.
Manchado E, Guillamot M, Malumbres M. Killing cells by targeting mitosis. Cell Death Differ. 2012;19:369–377.
Acknowledgments
This work was supported by the Natural Science Foundation of China (No. 81472272) and Natural Youth Foundation of China (No. 81401985).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
None.
Additional information
Huiyuan Qiu and Xiubing Zhang have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Qiu, H., Zhang, X., Ni, W. et al. Expression and Clinical Role of Cdc5L as a Novel Cell Cycle Protein in Hepatocellular Carcinoma. Dig Dis Sci 61, 795–805 (2016). https://doi.org/10.1007/s10620-015-3937-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3937-9