Abstract
Background
Despite the availability of safe and effective direct-acting antiviral drugs (DAAs), the vast majority of patients with chronic hepatitis C (HCV) in the USA remain untreated, in part due to lack of access to specialist providers.
Aims
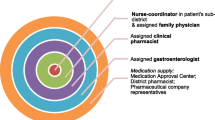
To determine the effectiveness of DAA-based treatment in medically underserved areas in California, in a healthcare model dependent on task-shifting—wherein a visiting hepatologist assesses patients for treatment eligibility, but subsequent routine follow-up evaluation of patients prescribed treatment is devolved to a part-time licensed vocational nurse under remote supervision of the hepatologist.
Methods
We retrospectively determined rates of sustained virologic response 12 weeks after treatment completion (SVR-12), adverse events, and treatment discontinuations in patients who received sofosbuvir-based DAA regimens between December 2013 and November 2014.
Results
Despite limited specialist provider involvement in medically underserved areas, all but two of 58 patients completed treatment, and 88 % of patients achieved the curative endpoint of undetectable HCV RNA 12 weeks after completing treatment (sustained virologic response, SVR-12). Almost 80 % of patients with cirrhosis and 85 % of patients with prior treatment experience achieved SVR-12.
Conclusions
Treatment effectiveness with sofosbuvir-based regimens in medically underserved areas utilizing task-shifting from a specialist to a mid-level provider is comparable to those achieved in pivotal clinical trials for these regimens, and to “real-world” experiences of tertiary care centers in the USA.

Similar content being viewed by others
References
Hepatitis and Liver Cancer. A national strategy for prevention and control of hepatitis B and C. Washington, DC: Institute of Medicine; 2010.
Denniston MM, Jiles RB, Drobeniuc J, et al. Chronic hepatitis C virus Infection in the United States, National Health and Nutrition Examination Survey 2003 to 2010. Ann. Intern. Med. 2014;160:293–300.
Reau N. HCV testing and linkage to care: expanding access: HCV testing and linkage to care. Clin. Liver Dis. 2014;4:31–34.
Kredo T, Adeniyi FB, Bateganya M, Pienaar ED. Task shifting from doctors to non-doctors for initiation and maintenance of antiretroviral therapy. In: The Cochrane Collaboration, ed. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd, Chichester. doi:10.1002/14651858.CD007331.pub3. Accessed 12 Dec 2014.
American Association for the Study of Liver Diseases, Infectious Diseases Society of America. Recommendations for testing, managing, and treating hepatitis C. http://www.hcvguidelines.org/printpdf/92. Accessed 5 Dec 2014.
Chirikov VV, Shaya FT, Howell CD. Contextual analysis of determinants of late diagnosis of hepatitis C virus infection in Medicare patients. Hepatology. 2015;62:68–78.
Zibbell JE, Iqbal K, Patel RC, et al. Increases in hepatitis C virus infection related to injection drug use among persons aged ≤ 30 years—Kentucky, Tennessee, Virginia, and West Virginia, 2006–2012. MMWR Morb. Mortal. Wkly. Rep. 2015;64:453–458.
Volk ML, Tocco R, Saini S, Lok ASF. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology. 2009;50:1750–1755.
Yehia BR, Schranz AJ, Umscheid CA, Lo Re V. The treatment cascade for chronic hepatitis C virus infection in the United States: a systematic review and meta-analysis. PLoS ONE. 2014;9:e101554.
Chhatwal J, Kanwal F, Roberts MS, Dunn MA. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann. Intern. Med. 2015;162:397.
Najafzadeh M, Andersson K, Shrank WH, et al. Cost-effectiveness of novel regimens for the treatment of hepatitis C Virus. Ann. Intern. Med. 2015;162:407.
Arora S, Thornton K, Murata G, et al. Outcomes of treatment for hepatitis C virus infection by primary care providers. N. Engl. J. Med. 2011;364:2199–2207.
Rongey C, Shen H, Hamilton N, Backus LI, Asch SM, Knight S. Impact of rural residence and health system structure on quality of liver care. PLoS ONE. 2013;8:e84826.
Lawitz E, Sulkowski MS, Ghalib R, et al. Simeprevir plus sofosbuvir, with or without ribavirin, to treat chronic infection with hepatitis C virus genotype 1 in non-responders to pegylated interferon and ribavirin and treatment-naive patients: the COSMOS randomised study. The Lancet. 2014;384:1756–1765.
Lawitz E, Mangia A, Wyles D, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 2013;368:1878–1887.
Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N. Engl. J. Med. 2014;370:1993–2001.
Jacobson IM, Gordon SC, Kowdley KV, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 2013;368:1867–1877.
Jensen DM, O’Leary J, Pockros P, et al. Safety and efficacy of sofosbuvir-containing regimens for hepatitis C: Real-world experience in a diverse, longitudinal observational cohort (HCV-TARGET). The Liver Meeting: American Association for the Study of Liver Diseases, Boston, MA. Abstract 45: 2014.
Backus LI, Belperio PS, Shahoumian TA, Loomis TP, Mole LA. Effectiveness of sofosbuvir-based regimens in genotype 1 and 2 hepatitis C virus infection in 4026 U.S. Veterans. Aliment. Pharmacol. Ther. 2015;42:559–573.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Rights and permissions
About this article
Cite this article
Jayasekera, C.R., Perumpail, R.B., Chao, D.T. et al. Task-Shifting: An Approach to Decentralized Hepatitis C Treatment in Medically Underserved Areas. Dig Dis Sci 60, 3552–3557 (2015). https://doi.org/10.1007/s10620-015-3911-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3911-6