Abstract
Background
To date, major hepatectomy with obstructive jaundice is still a highly risky and difficult surgery because of the high rate of complications. An excessive inflammatory response may be the primary hindrance to postoperative recovery of liver function.
Aims
Recent research has demonstrated that ulinastatin blocks the release of inflammatory factors and prevents the cytokine cascade reaction. This study was conducted to investigate the effect of ulinastatin on major hepatectomy after obstructive jaundice and to explore the potential mechanisms of this effect.
Methods
Male Sprague–Dawley rats were divided into three groups: sham, control and treated groups. In the control and treated groups, obstructive jaundice was induced, and a 70 % major hepatectomy was performed with implementation of ulinastatin treatment in the treated group but not the control group. The rats were sacrificed after hepatectomy on day 1, day 3, day 5 and day 7. The survival time, liver function, inflammatory cytokine expression and the indices of proliferation activities were examined. Kupffer cells were isolated, and the mRNA and protein levels of CD14 and NF-κB P65 in the Kupffer cells were determined.
Results
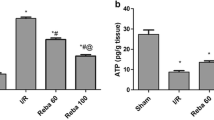
Compared to the control group, the survival rates, postoperative liver function, and the indices of proliferation activities were better in the treated group; in the treated group serum TNF-α and IL-6 levels were lower whereas serum IL-10 levels were higher. The expression of CD14 and NF-κB P65 in Kupffer cells at both the mRNA and protein levels was significantly higher in the control group than in the treated group.
Conclusions
Ulinastatin has a protective effect in major hepatectomy with obstructive jaundice by inhibiting Kupffer cell activation and modulating the hepatic cytokine response.






Similar content being viewed by others
References
Tsao JI, Nimura Y, Kamiya J, et al. Management of hilar cholangiocarcinoma: comparison of an American and a Japanese experience. Ann Surg. 2000;232:166–174.
Kondo S, Takada T, Miyazaki M, et al. Guidelines for the management of biliary tract and ampullary carcinomas: surgical treatment. J Hepato-Biliary-Pancreat Surg. 2008;15:41–54.
Benzoni E, Molaro R, Cedolini C, et al. Liver resection for HCC: analysis of causes and risk factors linked to postoperative complications. Hepatogastroenterology. 2007;54:186–189.
Jin S, Fu Q, Wuyun G, Wuyun T. Management of post-hepatectomy complications. World J Gastroenterol. 2013;19:7983–7991.
Deitch EA, Sittig K, Li M, Berg R, Specian RD. Obstructive jaundice promotes bacterial translocation from the gut. Am J Surg. 1990;159:79–84.
Sheen-Chen SM, Ho HT, Chia-Pei L, Hung KS, Eng HL. The effect of insulin-like growth factor-I on hepatocyte apoptosis after bile duct ligation in rat. Dig Dis Sci. 2006;51:2220–2224.
Pulitano C, Crawford M, Joseph D, Aldrighetti L, Sandroussi C. Preoperative assessment of postoperative liver function: the importance of residual liver volume. J Surg Oncol. 2014;110:445–450.
Liu F, Li Y, Wei Y, Li B. Preoperative biliary drainage before resection for hilar cholangiocarcinoma: whether or not? A systematic review. Dig Dis Sci. 2011;56:663–672.
Rerknimitr R, Angsuwatcharakon P, Ratanachu-ek T, et al. Asia-Pacific consensus recommendations for endoscopic and interventional management of hilar cholangiocarcinoma. J Gastroenterol Hepatol. 2013;28:593–607.
Makino H, Shimizu H, Ito H, et al. Changes in growth factor and cytokine expression in biliary obstructed rat liver and their relationship with delayed liver regeneration after partial hepatectomy. World J Gastroenterol. 2006;12:2053–2059.
Fujiwara Y, Shimada M, Yamashita Y, et al. Cytokine characteristics of jaundice in mouse liver. Cytokine. 2001;13:188–191.
Badger SA, Jones C, McCaigue M, et al. Cytokine response to portal endotoxaemia and neutrophil stimulation in obstructive jaundice. Eur J Gastroenterol Hepatol. 2012;24:25–32.
Vollmar B, Menger MD. The hepatic microcirculation: mechanistic contributions and therapeutic targets in liver injury and repair. Physiol Rev. 2009;89:1269–1339.
Yang YL, Li JP, Xu XP, Dou KF, Yue SQ, Li KZ. Protective effects of tumor necrosis factor alpha antibody and ulinastatin on liver ischemic reperfusion in rats. World J Gastroenterol. 2004;10:3161–3164.
Lu J, Chen YP, Wan R, Guo CY, Wang XP. Protective effects of ulinastatin on acute liver failure induced by lipopolysaccharide/d-galactosamine. Dig Dis Sci. 2012;57:399–404.
Higgins GM, Anderson RM. Experimental pathology of the liver: restoration of the liver on the white rat following partial surgical removal. Arch Pathol. 1931.
dos Santos JS, Kemp R, de Andrade MF, Neder L. Influence of biliary anastomosis on recovery from secondary biliary cirrhosis. Eur J Gastroenterol Hepatol. 2012;24:1039–1050.
Gong JP, Wu CX, Liu CA, et al. Intestinal damage mediated by Kupffer cells in rats with endotoxemia. World J Gastroenterol. 2002;8:923–927.
Dawiskiba J, Kornafel P, Kwiatkowska D, Zimecki M. Alterations of tumor necrosis factor-alpha and interleukin 6 production and activity of the reticuloendothelial system in experimental obstructive jaundice in rats. HPB. 2002;4:11–19.
Diamond T, Dolan S, Thompson RL, Rowlands BJ. Development and reversal of endotoxemia and endotoxin-related death in obstructive jaundice. Surgery. 1990;108:370–374.; discussion 374–375.
Maraslioglu M, Oppermann E, Blattner C, et al. Chronic ethanol feeding modulates inflammatory mediators, activation of nuclear factor-kappaB, and responsiveness to endotoxin in murine Kupffer cells and circulating leukocytes. Mediators Inflamm. 2014;2014:808695.
Onder A, Kapan M, Yuksel H, et al. The effects of erythropoietin on bacterial translocation and inflammation in rats with obstructive jaundice. Ann Ital Chir. 2014;85:159–165.
Yang LQ, Song JC, Irwin MG, Song JG, Sun YM, Yu WF. A clinical prospective comparison of anesthetics sensitivity and hemodynamic effect among patients with or without obstructive jaundice. Acta Anaesthesiol Scand. 2010;54:871–877.
Pergel A, Zengin K, Cercel A, Aki H, Kaya S. The effects of somatostatin and ursodeoxycholic acid in preventing the ischemic injury of the liver following Pringle maneuver in obstructive jaundice-rat model. Hepatogastroenterology. 2007;54:229–233.
Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34.
Varfolomeev EE, Ashkenazi A. Tumor necrosis factor: an apoptosis JuNKie? Cell. 2004;116:491–497.
Muratori L, Longhi MS. The interplay between regulatory and effector T cells in autoimmune hepatitis: implications for innovative treatment strategies. J Autoimmun. 2013;46:74–80.
Mesquida M, Leszczynska A, Llorenc V, Adan A. Interleukin-6 blockade in ocular inflammatory diseases. Clin Exp Immunol. 2014;176:301–309.
Zhang LJ, Wang XZ. Interleukin-10 and chronic liver disease. World J Gastroenterol. 2006;12:1681–1685.
Yoshidome H, Kato A, Edwards MJ, Lentsch AB. Interleukin-10 suppresses hepatic ischemia/reperfusion injury in mice: implications of a central role for nuclear factor kappaB. Hepatology (Baltimore, MD). 1999;30:203–208.
Smith K. Liver disease: Kupffer cells regulate the progression of ALD and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:503.
Dixon LJ, Barnes M, Tang H, Pritchard MT, Nagy LE. Kupffer cells in the liver. Compr Physiol. 2013;3:785–797.
Roberts RA, Ganey PE, Ju C, Kamendulis LM, Rusyn I, Klaunig JE. Role of the Kupffer cell in mediating hepatic toxicity and carcinogenesis. Toxicol Sci. 2007;96:2–15.
Okaya T, Lentsch AB. Cytokine cascades and the hepatic inflammatory response to ischemia and reperfusion. J Invest Surg. 2003;16:141–147.
Kolios G, Valatas V, Kouroumalis E. Role of Kupffer cells in the pathogenesis of liver disease. World J Gastroenterol. 2006;12:7413–7420.
Paik YH, Schwabe RF, Bataller R, Russo MP, Jobin C, Brenner DA. Toll-like receptor 4 mediates inflammatory signaling by bacterial lipopolysaccharide in human hepatic stellate cells. Hepatology (Baltimore, MD). 2003;37:1043–1055.
Mustafa SB, Flickinger BD, Olson MS. Suppression of lipopolysaccharide-induced nitric oxide synthase expression by platelet-activating factor receptor antagonists in the rat liver and cultured rat Kupffer cells. Hepatology (Baltimore, MD). 1999;30:1206–1214.
Hsieh WT, Liu YT, Lin WC. Anti-inflammatory properties of Ajuga bracteosa in vivo and in vitro study and their effects on mouse model of liver fibrosis. J Ethnopharmacol. 2011;135:116–125.
Acknowledgments
This work was supported by the Science and Technology Planning Project of Guangdong Province (No. 2012B061700105), China.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Xun Li and Jing Li have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Li, X., Li, J., Ou, YJ. et al. Hepatoprotective Effect of Ulinastatin in a Rat Model of Major Hepatectomy After Obstructive Jaundice. Dig Dis Sci 60, 1680–1689 (2015). https://doi.org/10.1007/s10620-015-3543-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-015-3543-x