Abstract
Background and Aims
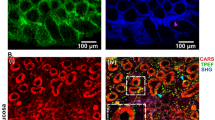
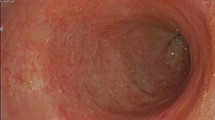
The intestinal epithelial barrier plays an important role in the pathogenesis of non-steroidal anti-inflammatory drug-induced enteropathy, and its disruption is often associated with increased cell shedding. The purpose of this report is to observe the gap density in indomethacin-induced small intestinal damage by confocal laser endomicroscopy (CLE) and to investigate the mechanisms involved in this process and how mucosal protectants improve intestinal epithelial barrier dysfunction. CLE is expected to provide a new way for evaluating non-steroidal anti-inflammatory drugs-induced enteropathy in humans and assessing drug efficacy.
Methods
Using the new technique of CLE, we established a method to evaluate, in real time, intestinal damage after the administration of indomethacin in Wistar rats by investigating the gap density in the small intestine. The mucosal protectant teprenone and acid-suppressant rabeprazole were then given by gavage before and after the administration of indomethacin, and the mechanisms affecting the intestinal epithelial barrier were investigated.
Results
Using CLE, gaps could be clearly observed and easily distinguished from goblet cells. Gap density was increased after the administration of indomethacin. During this process, the expression of tumor necrosis factor-α, nuclear factor-κB, and caspase-3 was up-regulated and the expression of tight junctions was down-regulated, which led to the damage of the epithelial barrier. Teprenone and rabeprazole could intervene in this pathway and protect the integrity of the epithelial barrier.
Conclusions
CLE can be objective, accurate, and real time in investigating gap density. Teprenone and rabeprazole can prevent indomethacin-induced intestinal lesions and protect the epithelial barrier by intervening in the tumor necrosis factor-α pathway. Gap density was expected to be an indicator of evaluating intestinal inflammation and drug efficacy.






Similar content being viewed by others
References
Brooks P, Emery P, Evans J, et al. Interpreting the clinical significance of the differential inhibition of COX-1 and COX-2. Rheumatology. 1999;38:779–788.
Bedson J, Whitehurst T, Lewis M, et al. Factors affecting over-the-counter use of aspirin in the secondary prophylaxis of cardiovascular disease. Br J Gen Pract. 2001;51:1001–1003.
Brunelli C, Amici C, Angelini M, et al. The nonsteroidal anti-inflammatory drug indomethacin activates the eIF2 alpha kinase PKR, causing a translational block in human colorectal cancer cells. Biochem J. 2012;443:379–386.
Brady RR, Loveridge CJ, Dunlop MG, Stark LA. c-Src dependency of NSAID-induced effects on NF-κB-mediated apoptosis in colorectal cancer cells. Carcinogenesis. 2011;32:1069–1077.
Maiden L, Thjodleifsson B, Theodors A, et al. A quantitative analysis of NSAID-induced small bowel pathology by capsule enteroscopy. Gastroenterology. 2005;128:1172–1178.
Saurin JC. Capsule endoscopy. Endoscopy. 2007;39:986–991.
Matsumoto T, Kudo T, Esaki M, et al. Prevalence of non-steroidal anti-inflammatory drug-induced enteropathy determined by double-balloon endoscopy: a Japanese multicenter study. Scand J Gastroenterol. 2008;43:490–496.
Graham DY, Opekun AR, Willingham FF, et al. Visible small intestinal mucosal injury in chronic NSAID users. Clin Gastroenterol Hepatol. 2005;3:55–59.
Adebayo D, Bjarnason I. Is non-steroidal anti-inflammatory drug (NSAID) enteropathy clinically more important than NSAID gastropathy? Postgrad Med J. 2006;82:186–191.
Fortun PJ, Hawkey CJ. Nonsteroidal anti-inflammatory drugs and the small intestine. Curr Opin Gastroenterol. 2007;23:134–141.
Watson AJ, Chu S, Sieck L, et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912.
Kiesslich R, Goetz M, Angus EM, et al. Identification of epithelial gaps in human small and large intestine by confocal endomicroscopy. Gastroenterology. 2007;133:1769–1778.
Bullen TF, Forrest S, Campbell F, et al. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–1063.
Garrett WS, Lord GM, Punit S, et al. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45.
Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561.
Liu JJ, Wong K, Thiesen AL, et al. Increased epithelial gaps in the small intestines of patients with inflammatory bowel disease: density matters. Gastrointest Endosc. 2011;73:1174–1180.
Liu JJ, Madsen KL, Boulanger P, et al. Mind the gaps: confocal endomicroscopy showed increased density of small bowel epithelial gaps in inflammatory bowel disease. J Clin Gastroenterol. 2011;45:240–245.
Liu JJ, Rudzinski JK, Mah SJ, et al. Epithelial gaps in a rodent model of inflammatory bowel disease: a quantitative validation study. Clin Transl Gastroenterol. 2011;2:e3.
Jobin C, Sartor RB. The IκB/NF-κB system: a key determinant of mucosal inflammation and protection. Am J Physiol Cell Physiol. 2000;278:C451–C462.
Baeuerle P, Henkle T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179.
Barnes P, Karin M. Nuclear factor-κB, a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071.
Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–G475.
Gitter AH, Bendfeldt K, Sehmitz H, et al. Epithelial barrier defects in HT-29/B6 colonic cell monolayers induced by tumor necrosis factor-alpha. Ann N Y Acad Sci. 2000;915:193–203.
Ma TY, lwamoto GK, Hoa NT, et al. TNF-alpha-induced increase in intestinal epithelial tight junction permeability requires NF-kappa B activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:367–376.
Marano CW, Lewis SA, Garulacan LA, et al. Tumor necrosis factor-alpha increases sodium and chloride conductance across the right junction of CACO-2 BBE, a human intestinal epithelial cell line. J Membr Biol. 1998;161:263–274.
Fukui A, Naito Y, Handa O, et al. Acetyl salicylic acid induces damage to intestinal epithelial cells by xidation-related modifications of ZO-1. Am J Physiol Gastrointest Liver Physiol. 2012;303:G927–G936.
Watson AJ, Chu S, Sieck L, et al. Epithelial barrier function in vivo is sustained despite gaps in epithelial layers. Gastroenterology. 2005;129:902–912.
Pozzoli C, Menozzi A, Grandi D, et al. Protective effects of proton pump inhibitors against indomethacin-induced lesions in the rat small intestine. Naunyn Schmiedebergs Arch Pharmacol. 2007;374:283–291.
Yoda Y, Amagase K, Kato S, et al. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin-induced small intestinal ulceration in rats through induction of hemeoxygenase-1. J Physiol Pharmacol. 2010;61:287–294.
Lim YJ, Phan TM, Dial EJ, et al. In vitro and in vivo protection against indomethacin-induced small intestinal injury by proton pump inhibitors, acid pump antagonists, or indomethacin-phosphatidylcholine. Digestion. 2012;86:171–177.
Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322.
Acknowledgments
No financial support was received for this work from any company. This study was supported by a Natural Science Foundation Committee project (NSFC81170352).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, S., Wang, H., Gao, H. et al. Increased Gap Density Predicts Weakness of the Epithelial Barrier In Vivo by Confocal Laser Endomicroscopy in Indomethacin-Induced Enteropathy. Dig Dis Sci 59, 1398–1405 (2014). https://doi.org/10.1007/s10620-014-3076-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-014-3076-8