Abstract
Background
Hypoxia is often found in solid tumors and is associated with tumor progression and poor clinical outcomes. We elucidated the mechanism by which netrin-1 released under hypoxic stress can induce epithelial–mesenchymal transition (EMT) to promote invasion in hepatocellular carcinoma (HCC) cells.
Methods
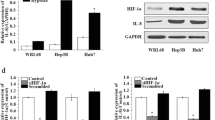
The expression of netrin-1 and the dependent receptors UNC5H and deleted in colorectal cancer (DCC) in HCC was examined by immunohistochemistry or western blot. The HepG2 cells were cultured in 21 % O2 (normoxia) or 1 % O2 (hypoxia) for 24 h. The release of netrin-1 from hypoxic cells was detected by ELISA. Expression of E-cadherin and vimentin were examined by western blot. Inverted microscopy or confocal microscopy was used to show the cell morphology or cytoskeletal rearrangements. Cell invasion induced by hypoxia was analyzed by Transwell chamber. Cytokine IL-8 and IL-10 mRNA levels were assessed by real-time PCR.
Results
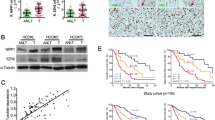
The expression of netrin-1 was increased in HCC tissue and cell lines. The dependent receptors UNC5H and DCC were decreased in most HCC cell lines. Hypoxia induced netrin-1 release in a time-dependent manner. EMT induction was found to occur in hypoxic HCC cells in a process that was dependent on the extracellular release of netrin-1. Moreover, overexpression of netrin-1 resulted in EMT induction in normoxic tumor cells. Cytoskeletal rearrangements were found to occur and cell invasion was increased in cells with netrin-1 overexpression. Lastly, mRNA of IL-8 and IL-10 were also increased after recombinant human netrin-1 treatment.
Conclusion
These results suggest that in hypoxic HCC cells, netrin-1 activates downstream signaling pathways to induce EMT activation with subsequent production of multiple inflammatory mediators which in turn promotes cancer invasion.





Similar content being viewed by others
References
Harrison L, Blackwell K. Hypoxia and anemia: factors in decreased sensitivity to radiation therapy and chemotherapy? Oncologist. 2004;9:31–40.
Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29:285–293.
Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44.
Peinado H, Lavotshkin S, Lyden D. The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol. 2011;21:139–146.
Yan W, Chang Y, Liang X, et al. High-mobility group box 1 activates caspase-1 and promotes hepatocellular carcinoma invasiveness and metastases. Hepatology. 2012;55:1863–1875.
Lee JM, Dedhar S, Kalluri R, Thompson TW. The epithelial–mesenchymal transition: new insights in signaling, development, and disease. J Cell Biol. 2006;172:973–981.
Yan W, Fu Y, Tian D, et al. PI3 kinase/Akt signaling mediates epithelial–mesenchymal transition in hypoxic hepatocellular carcinoma cells. Biochem Biophys Res Commun. 2009;382:631–636.
Forcet C, Stein E, Pays L, et al. Netrin-1-mediated axon outgrowth requires deleted in colorectal cancer-dependent MAPK activation. Nature. 2002;417:443–447.
Furne C, Rama N, Corset V, Chedotal A, Mehlen P. Netrin-1 is a survival factor during commissural neuron navigation. Proc Natl Acad Sci USA. 2008;105:14465–14470.
Paradisi A, Mehlen P. Netrin-1, a missing link between chronic inflammation and tumor progression. Cell Cycle. 2010;9:1253–1262.
Paradisi A, Maisse C, Coissieux MM, et al. Netrin-1 up-regulation in inflammatory bowel diseases is required for colorectal cancer progression. Proc Natl Acad Sci USA. 2009;106:17146–17151.
Fitamant J, Guenebeaud C, Coissieux MM, et al. Netrin-1 expression confers a selective advantage for tumor cell survival in metastatic breast cancer. Proc Natl Acad Sci USA. 2008;105:4850–4855.
Delloye-Bourgeois C, Brambilla E, Coissieux MM, et al. Interference with netrin-1 and tumor cell death in non-small cell lung cancer. J Natl Cancer Inst. 2009;101:237–247.
Delloye-Bourgeois C, Fitamant J, Paradisi A, et al. Netrin-1 acts as a survival factor for aggressive neuroblastoma. J Exp Med. 2009;206:833–847.
Mazelin L, Bernet A, Bonod-Bidaud C, et al. Netrin-1 controls colorectal tumorigenesis by regulating apoptosis. Nature. 2004;431:80–84.
Mehlen P, Guenebeaud C. Netrin-1 and its dependence receptors as original targets for cancer therapy. Curr Opin Oncol. 2010;22:46–54.
Serafini T, Kennedy TE, Galko MJ, Mirzayan C, Jessell TM, Tessier-Lavigne M. The netrins define a family of axon outgrowth-promoting proteins homologous to C. elegans UNC-6. Cell. 1994;78:409–424.
Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons in the embryonic spinal cord. Cell. 1994;78:425–435.
Meyerhardt JA, Caca K, Eckstrand BC, et al. Netrin-1: interaction with deleted in colorectal cancer (DCC) and alterations in brain tumors and neuroblastomas. Cell Growth Differ. 1999;10:35–42.
Grunert S, Jechlinger M, Beug H. Diverse cellular and molecular mechanisms contribute to epithelial plasticity and metastasis. Nat Rev Mol Cell Biol. 2003;4:657–665.
Voorzanger N, Touitou R, Garcia E, et al. Interleukin (IL)-10 and IL-6 are produced in vivo by non-Hodgkin’s lymphoma cells and act as cooperative growth factors. Cancer Res. 1996;56:5499–5505.
Rampone B, Schiavone B, Confuorto G. Current management of hepatocellular cancer. Curr Oncol Rep. 2010;12:186–192.
Jarnagin W, Chapman WC, Curley S, et al. Surgical treatment of hepatocellular carcinoma: expert consensus statement. HPB (Oxford). 2010;12:302–310.
Gwak GY, Yoon JH, Kim KM, Lee HS, Chung JW, Gores GJ. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol. 2005;42:358–364.
Chia SK, Wykoff CC, Watson PH, et al. Prognostic significance of a novel hypoxia-regulated marker, carbonic anhydrase IX, in invasive breast carcinoma. J Clin Oncol. 2001;19:3660–3668.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454.
Imai T, Horiuchi A, Wang C, et al. Hypoxia attenuates the expression of E-cadherin via up-regulation of SNAIL in ovarian carcinoma cells. Am J Pathol. 2003;163:1437–1447.
Lester RD, Jo M, Montel V, Takimoto S, Gonias SL. uPAR induces epithelial–mesenchymal transition in hypoxic breast cancer cells. J Cell Biol. 2007;178:425–436.
Krishnamachary B, Zagzag D, Nagasawa H, et al. Hypoxia-inducible factor-1-dependent repression of E-cadherin in von Hippel-Lindau tumor suppressor-null renal cell carcinoma mediated by TCF3, ZFHX1A, and ZFHX1B. Cancer Res. 2006;66:2725–2731.
Hong K, Nishiyama M, Henley J, Tessier-Lavigne M, Poo M. Calcium signalling in the guidance of nerve growth by netrin-1. Nature. 2000;403:93–98.
Mehlen P, Delloye-Bourgeois C, Chedotal A. Novel roles for Slits and netrins: axon guidance cues as anticancer targets? Nat Rev Cancer. 2011;11:188–197.
Acknowledgments
This study is supported by the National Natural Science Foundation of China (No. 81000928 and No. 81000159).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yan, W., Han, P., Zhou, Z. et al. Netrin-1 Induces Epithelial–Mesenchymal Transition and Promotes Hepatocellular Carcinoma Invasiveness. Dig Dis Sci 59, 1213–1221 (2014). https://doi.org/10.1007/s10620-013-3016-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-3016-z