Abstract
Background
Thiopurine S-methyltransferase (TPMT) is a key enzyme that deactivates thiopurines, into their inactive metabolite, 6-methylmercaptopurine. Intermediate and low TPMT activity may lead to leukopenia following thiopurine treatment. The aim of this study was to determine TPMT activity and TPMT alleles (genotype–phenotype correlation) in Jews, aiming to develop an evidence-based pharmacogenetic assay for this population.
Methods
TPMT activity was determined in 228 Jewish volunteers by high performance liquid chromatography. Common allelic variants in the Caucasian population [TPMT*2 (G238C), TPMT *3A (G460A and A719G), TPMT* 3B (G460A) and TPMT*3C (A719G)] were tested. Phenotype–genotype correlation was examined and discordant cases were fully sequenced to identify novel genetic variants.
Results
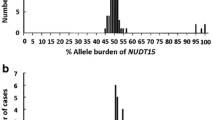
Mean TPMT activity was 15.4 ± 4 U/ml red blood cells (range 1–34). Intermediate activity was found in 33/228 (14 %) subjects and absent activity was found in one sample (0.4 %). Only eight individuals (3.5 % of the entire cohort and 24 % of those with intermediate/low activity) were identified as carriers of a TPMT genetic variant, all of whom had the TPMT*3A allele. Sequencing the entire TPMT coding region and splice junctions in the remainder of the discordant cases did not reveal any novel variants.
Conclusion
Genotyping TPMT in Jews yields a much lower rate of variants than identified in the general Caucasian population. We conclude that a biochemical assay to determine TPMT enzymatic activity should be performed in Jews before starting thiopurine treatment in order to identify low activity subjects.

Similar content being viewed by others
References
Osterman MT, Kundu R, Lichtenstein GR, Lewis JD. Association of 6-thioguanine nucleotide levels and inflammatory bowel disease activity: a meta-analysis. Gastroenterology. 2006;130:1047–1053.
Schutz E, Gummert J, Mohr FW, Armstrong VW, Oellerich M. Should 6-thioguanine nucleotides be monitored in heart transplant recipients given azathioprine? Ther Drug Monit. 1996;18:228–233.
Yenson PR, Forrest D, Schmiegelow K, Dalal BI. Azathioprine-associated acute myeloid leukemia in a patient with Crohn’s disease and thiopurine S-methyltransferase deficiency. Am J Hematol. 2008;83:80–83.
Nygaard U, Toft N, Schmiegelow K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin Pharmacol Ther. 2004;75:274–281.
Sahasranaman S, Howard D, Roy S. Clinical pharmacology and pharmacogenetics of thiopurines. Eur J Clin Pharmacol. 2008;64:753–767.
Armstrong L, Sharif JA, Galloway P, McGrogan P, Bishop J, Russell RK. Evaluating the use of metabolite measurement in children receiving treatment with a thiopurine. Aliment Pharmacol Ther. 2011;34:1106–1114.
Tai HL, Krynetski EY, Schuetz EG, Yanishevski Y, Evans WE. Enhanced proteolysis of thiopurine S-methyltransferase (TPMT) encoded by mutant alleles in humans (TPMT*3A, TPMT*2): mechanisms for the genetic polymorphism of TPMT activity. Proc Natl Acad Sci USA. 1997;94:6444–6449.
McLeod HL, Siva C. The thiopurine S-methyltransferase gene locus—implications for clinical pharmacogenomics. Pharmacogenomics.. 2002;3:89–98.
Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–662.
Ansari A, Hassan C, Duley J, et al. Thiopurine methyltransferase activity and the use of azathioprine in inflammatory bowel disease. Aliment Pharmacol Ther. 2002;16:1743–1750.
Seidman EG. Clinical use and practical application of TPMT enzyme and 6-mercaptopurine metabolite monitoring in IBD. Rev Gastroenterol Disord.. 2003;3(Suppl 1):S30–S38.
van Egmond R, Chin P, Zhang M, Sies CW, Barclay ML. High TPMT enzyme activity does not explain drug resistance due to preferential 6-methylmercaptopurine production in patients on thiopurine treatment. Aliment Pharmacol Ther. 2012;35:1181–1189.
Cuffari C, Dassopoulos T, Turnbough L, Thompson RE, Bayless TM. Thiopurine methyltransferase activity influences clinical response to azathioprine in inflammatory bowel disease. Clin Gastroenterol Hepatol.. 2004;2:410–417.
Dubinsky MC, Reyes E, Ofman J, Chiou CF, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn’s disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239–2247.
Hindorf U, Lindqvist M, Peterson C, et al. Pharmacogenetics during standardised initiation of thiopurine treatment in inflammatory bowel disease. Gut. 2006;55:1423–1431.
Hindorf U, Appell ML. Genotyping should be considered the primary choice for pre-treatment evaluation of thiopurine methyltransferase function. J Crohns Colitis.. 2012;6:655–659.
Boson WL, Romano-Silva MA, Correa H, Falcaõ RP, Teixeira-Vidigal PV, De Marco L. Thiopurine methyltransferase polymorphisms in a Brazilian population. Pharmacogenomics J.. 2003;3:178–182.
Tai HL, Krynetski EY, Yates CR, et al. Thiopurine S-methyltransferase deficiency: two nucleotide transitions define the most prevalent mutant allele associated with loss of catalytic activity in Caucasians. Am J Hum Genet. 1996;58:694–702.
Lowenthal A, Meyerstein N, Ben-Zvi Z. Thiopurine methyltransferase activity in the Jewish population of Israel. Eur J Clin Pharmacol. 2001;57:43–46.
Fevery J, Henckaerts L, Van Oirbeek R, et al. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int.. 2012;32:214–222.
Ronen O, Cohen SB, Rund D. Evaluating frequencies of thiopurine S-methyl transferase (TPMT) variant alleles in Israeli ethnic subpopulations using DNA analysis. Isr Med Assoc J.. 2010;12:721–725.
Khalil MN, Erb N, Khalil PN, Escherich G, Janka-Schaub GE. Interference free and simplyfied liquid chromatography-based determination of thiopurine S-methyltransferase activity in erythrocytes. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;821:105–111.
Eaton JE, Silveira MG, Pardi DS, et al. High-dose ursodeoxycholic acid is associated with the development of colorectal neoplasia in patients with ulcerative colitis and primary sclerosing cholangitis. Am J Gastroenterol. 2011;106:1638–1645.
Efrati E, Adler L, Krivoy N, Sprecher E. Distribution of TPMT risk alleles for thioupurine toxicity in the Israeli population. Eur J Clin Pharmacol. 2009;65:257–262.
Dewit O, Starkel P, Roblin X. Thiopurine metabolism monitoring: implications in inflammatory bowel diseases. Eur J Clin Invest. 40:1037–1047.
Priest VL, Begg EJ, Gardiner SJ, et al. Pharmacoeconomic analyses of azathioprine, methotrexate and prospective pharmacogenetic testing for the management of inflammatory bowel disease. Pharmacoeconomics.. 2006;24:767–781.
Winter J, Walker A, Shapiro D, Gaffney D, Spooner RJ, Mills PR. Cost-effectiveness of thiopurine methyltransferase genotype screening in patients about to commence azathioprine therapy for treatment of inflammatory bowel disease. Aliment Pharmacol Ther. 2004;20:593–599.
Marra CA, Esdaile JM, Anis AH. Practical pharmacogenetics: the cost effectiveness of screening for thiopurine s-methyltransferase polymorphisms in patients with rheumatological conditions treated with azathioprine. J Rheumatol. 2002;29:2507–2512.
Oh KT, Anis AH, Bae SC. Pharmacoeconomic analysis of thiopurine methyltransferase polymorphism screening by polymerase chain reaction for treatment with azathioprine in Korea. Rheumatology (Oxford). 2004;43:156–163.
van den Akker, van Marle ME, Gurwitz D, Detmar SBet al. Cost-effectiveness of pharmacogenomics in clinical practice: a case study of thiopurine methyltransferase genotyping in acute lymphoblastic leukemia in Europe. Pharmacogenomics 2006;7:783–792.
Donnan JR, Ungar WJ, Mathews M, Hancock-Howard RL, Rahman P. A cost effectiveness analysis of thiopurine methyltransferase testing for guiding 6-mercaptopurine dosing in children with acute lymphoblastic leukemia. Pediatric Blood Cancer. 2011;57:231–239.
Acknowledgments
This study was funded by internal academic grants from Shaare Zedek Medical Center and the Hebrew University of Jerusalem. There was no influence of the sponsors on the study design, implementation or analysis.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Yair Kasirer, Rephael Mevorach, Reeval Segel and Dan Turner contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kasirer, Y., Mevorach, R., Renbaum, P. et al. Thiopurine S-methyltransferase (TPMT) Activity Is Better Determined by Biochemical Assay Versus Genotyping in the Jewish Population. Dig Dis Sci 59, 1207–1212 (2014). https://doi.org/10.1007/s10620-013-3008-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-3008-z