Abstract
Background
The human thiamine transporter-2 (hTHTR-2) is involved in the intestinal absorption of thiamine. Recent studies with membrane transporters of other nutrients/substrates have shown that they have associated proteins that affect different aspects of their physiology and cell biology. Nothing is known about protein(s) that interact with hTHTR-2 in intestinal epithelial cells and influence its physiological function and/or its cell biology.
Aims
The aim of this study was to identify protein partner(s) that interact with hTHTR-2 in human intestinal cells and determine the physiological/biological consequence of that interaction.
Methods
The yeast split-ubiquitin two-hybrid approach was used to screen a human intestinal cDNA library. GST-pull-down and cellular co-localization approaches were used to confirm the interaction between hTHTR-2 and the associated protein(s). The effect of such an interaction on hTHTR-2 function was examined by 3H-thiamine uptake assays.
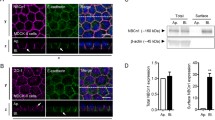
Results
Our screening results identified the human TransMembrane 4 SuperFamily 4 (TM4SF4) as a potential interactor with hTHTR-2. This interaction was confirmed by an in vitro GST-pull-down assay, and by live-cell confocal imaging of HuTu-80 cells co-expressing hTHTR-2–GFP and mCherry–TM4SF4 (the latter displayed a significant overlap of these two proteins in intracellular vesicles and at the cell membrane). Co-expression of hTHTR-2 with TM4SF4 in HuTu-80 cells led to a significant induction in thiamine uptake. In contrast, silencing TM4SF4 with gene-specific siRNA led to a significant decrease in thiamine uptake.
Conclusions
These results show for the first time that the accessory protein TM4SF4 interacts with hTHTR-2 and influences the physiological function of the thiamine transporter.




Similar content being viewed by others
References
Berdanier CD. Advanced Nutrition Micronutrients. Boca Raton, FL: CRC; 1998.
Tanphaichirt V. Thiamin. In: Shils ME, Olsen JA, Shike M, eds. Modern Nutrition in Health and Disease. New York: Lea and Febiger. 1994, pp. 359–375.
Victor M, Adams RD, Collins GH. The Wernicke-Korsakoff syndrome. A clinical and pathological study of 245 patients, 82 with post-mortem examinations. Philadelphia, PA: Davis; 1989.
Saito N, Kimura M, Kuchiba A, Itokawa Y. Blood thiamine levels in outpatients with diabetes mellitus. J Nutr Sci Vitaminol. 1987;33:421–430.
Tallaksen CME, Bohmer T, Bell H. Blood and serum thiamin and thiamin phosphate esters concentrations in patients with alcohol dependence syndrome before and after thiamin treatment. Alcohol Clin Exp Res. 1992;16:320–325.
Leevy CM, Baker H. Vitamins and alcoholism. Am J Clin Nutr. 1968;21:1325–1328.
Tomasulo PA, Kater RM, Iber FL. Impairment of thiamine absorption in alcoholism. Am J Clin Nutr. 1968;21:1341–1344.
Thomson AD. The absorption of radioactive sulphur-labelled thiamine hydrochloride in control subjects and in patients with intestinal malabsorption. Clin Sci. 1996;31:167–179.
Said HM. Intestinal absorption of water soluble vitamins in health and disease. Biochem J. 2011;437:357–372.
Fleming JC, Tartaglini E, Steinkamp M, et al. The gene mutated in thiamine-responsive anaemia with diabetes and deafness (TRMA) encodes a functional thiamine transporter. Nat Genet. 1999;22:305–308.
Rajagopal A, Edmondson A, Goldman D, Zhao R. SLC19A3 encodes a second thiamine transporter ThTr2. Biochim Biophys Acta. 2001;1537:175–178.
Said HM, Balamurugan K, Subramanian VS, Marchant JS. Expression and functional contribution of hTHTR-2 in thiamin absorption in human intestine. Am J Physiol Gastrointest Liver Physiol. 2004;286:G491–G498.
Boulware MJ, Subramanian VS, Said HM, Marchant JS. Polarized expression of members of the solute carrier SLC19A gene family of water-soluble multivitamin transporters: implications for physiological function. Biochem J. 2003;15:43–48.
Subramanian VS, Marchant JS, Said HM. Targeting and trafficking of the human thiamine transporter-2 in epithelial cells. J Biol Chem. 2006;281:5233–5245.
Ashokkumar B, Nabokina SM, Ma TY, Said HM. Identification of dynein light chain road block-1 as a novel interaction partner with the human reduced folate carrier. Am J Physiol Gastrointest Liver Physiol. 2009;297:G480–G487.
Nabokina SM, Subramanian VS, Said HM. Association of PDZ-containing protein PDZD11 with the human sodium-dependent multivitamin transporter. Am J Physiol Gastrointest Liver Physiol. 2011;300:G561–G567.
Nabokina SM, Senthilkumar SR, Said HM. Tspan-1 interacts with the thiamine transporter-1 in human intestinal epithelial cells and modulates its stability. Am J Physiol Gastrointest Liver Physiol. 2011;301:G808–G813.
Subramanian VS, Nabokina SM, Patton JR, et al. Glyoxalate reductase/hydroxypyruvate reductase interacts with the sodium-dependent vitamin C transporter-1 to regulate cellular vitamin C homeostasis. Am J Physiol Gastrointest Liver Physiol. 2013;304:G1079–G1086.
Sun AQ, Balasubramaniyan N, Liu CJ, Shahid M, Suchy FJ. Association of the 16-kDa subunit c of vacuolar proton pump with the ileal Na+-dependent bile acid transporter: protein–protein interaction and intracellular trafficking. J Biol Chem. 2004;279:16295–16300.
Ortiz DF, Moseley J, Calderon G, Swift AL, Li S, Arias IM. Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin–Darby canine kidney cells. J Biol Chem. 2004;279:32761–32770.
Munehira Y, Ohnishi T, Kawamoto S, et al. Alpha1-syntrophin modulates turnover of ABCA1. J Biol Chem. 2004;279:15091–15095.
Bartel PL, Chein CT, Sternglanz R, Fields S. Using the two-hybride system to detect protein–protein interactions. In: Hartley DA, ed. Cellular Interactions in Development: A Practical Approach. Oxford, UK: Oxford University Press; 1993:153–179.
Formsteecher E, Aresta S, Collura V, et al. Protein interaction mapping: A Drosphilia case study. Genome Res. 2005;15:376–384.
Fromont-Racine M, Rain JC, Legrain P. Toward a functional analysis of the yeast genome through exhaustive two-hybrid screens. Nat Genet. 1997;16:277–282.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408.
Subramanian VS, Marchant JS, Said HM. Biotin-responsive basal ganglia disease-linked mutations inhibit thiamine transport via hTHTR2: biotin is not a substrate for hTHTR2. Am J Physiol Cell Physiol. 2006;291:C851–C859.
Wice BM, Gordon JI. A tetraspan membrane glycoprotein produced in the human intestinal epithelium and liver that can regulate cell density-dependent proliferation. J Biol Chem. 1995;270:21907–21918.
Liu Z, Zhao M, Yokoyama KK, Li T. Molecular cloning of a cDNA for rat TM4SF4, a homolog of human il-TMP (TM4SF4), and enhanced expression of the corresponding gene in regenerating rat liver. Biochim Biophys Acta. 2001;1518:183–189.
Anderson KR, Singer RA, Balderes DA, et al. The L6 domain tetraspanin Tm4sf4 regulates endocrine pancreas differentiation and directed cell migration. Development. 2011;138:3213–3224.
Rindi G, Laforenza U. Thiamin intestinal transport and related issues: recent aspects. Pro Soc Exp Biol Med. 2000;224:246–255.
Li Y, Wang L, Qiu J, et al. Human tetraspanin transmembrane 4 superfamily member 4 or intestinal and liver tetraspan membrane protein is overexpressed in hepatocellular carcinoma and accelerates tumor cell growth. Acta Biochim Biophys Sin. 2012;44:224–232.
Acknowledgments
This study was supported by grants from the Department Veterans Affairs and the National Institute of Health (DK-56061-15).
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Subramanian, V.S., Nabokina, S.M. & Said, H.M. Association of TM4SF4 with the Human Thiamine Transporter-2 in Intestinal Epithelial Cells. Dig Dis Sci 59, 583–590 (2014). https://doi.org/10.1007/s10620-013-2952-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2952-y