Abstract
Background
Osteoprotegerin (OPG), a soluble member of the tumor necrosis factor (TNF) receptor super-family, is a key factor inhibiting the differentiation and activation of osteoclasts. It has recently been implicated as a disease marker for inflammatory bowel disease (IBD) yet its role in the intestinal epithelial inflammatory response remains unknown.
Aim
The primary objective of this study was to investigate whether OPG has a role in intestinal inflammation and a potential role in IBD pathogenesis.
Methods
Caco-2 and HT-29 cells were grown in vitro to confluence on culture-permeable supports and then co-cultured with either TNF-α or OPG. After exposure to either TNF-α or OPG, interleukin (IL)-8 protein and mRNA levels were evaluated. Ussing chamber, western blotting, real-time polymerase chain reaction, and immunofluorescence were used to further investigate the effect of OPG on intestinal barrier integrity and function.
Results
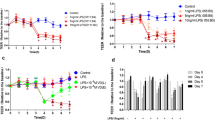
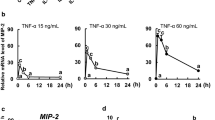
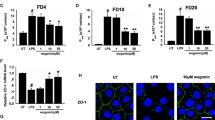
Similar to TNF-α, treatment of monolayers with OPG caused increased monolayer permeability and diminished tight junction function and integrity, with loss of tight junction proteins from cell membranes. This was accompanied by elevated IL-8 protein and gene levels (P < 0.05). Western blotting also revealed that OPG, similar to TNF-α, induced NF-κB activation, as shown by inhibition of NF-κB kinase subunit-α phosphorylation.
Conclusions
These results indicate that OPG has pro-inflammatory properties because it induces gut barrier dysfunction and secretion of other pro-inflammatory cytokines. These results also provide evidence that OPG is likely to exert its pro-inflammatory effects through NF-κB activation and may potently contribute to IBD pathogenesis.






Similar content being viewed by others
References
Forster C. Tight junctions and the modulation of barrier function in disease. Histochem Cell Biol. 2008;130:55–70.
McGilligan VE, Wallace JM, Heavey PM, Ridley DL, Rowland IR. Hypothesis about mechanisms through which nicotine might exert its effect on the interdependence of inflammation and gut barrier function in Ulcerative colitis. Inflamm Bowel Dis. 2007;13:108115.
Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin 2, 5 and 8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72.
Edelblum KL, Turner JR. The tight junction in inflammatory disease: communication breakdown. Curr Opin Pharmacol. 2009;9:715–720.
Reuter BK, Pizarro TT. Mechanisms of tight junction dysregulation in the SAMP1/YitFc model of Crohn’s disease-like ileitis. Ann N Y Acad Sci. 2009;1165:301–307.
Linskens RK, Huijsdens XW, Savelkoul PH, Vandenbroucke-Grauls CM, Meuwissen SG. The bacterial flora in inflammatory bowel disease: current insights in pathogenesis and the influence of antibiotics and probiotics. Scand J Gastroenterol Suppl. 2001;234:29–40.
Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205.
Wang F, Graham WV, Wang Y, Witkowski ED, Schwarz BT, Turner JR. Interferon-gamma and tumor necrosis factor-alpha synergize to induce intestinal epithelial barrier dysfunction by up-regulating myosin light chain kinase expression. Am J Pathol. 2005;166:409–419.
Yamamoto JK. Basic and clinical aspects of osteoporosis in inflammatory bowel disease. Wold J Gastroenterol. 2007;13:G6156–G6165.
Ma TY, Iwamoto GK, Hoa NT, et al. TNF-α-induced increase in intestinal epithelial tight junction permeability requires NF-κB activation. Am J Physiol Gastrointest Liver Physiol. 2004;286:G367–G376.
Bantel H, Berg C, Vieth M, et al. Mesalazine inhibits activation of transcription factor NF-B in inflamed mucosa of patients with ulcerative colitis. Am J Gastroenterol. 2000;95:3452–3457.
Schreiber S, Nikolaus S, Hampe J. Activation of nuclear factor kappa B inflammatory bowel disease. Gut. 1998;42:477–484.
Schottelius AJG, Baldwin-Jr AS. A role of transcription factor NF-κB in intestinal inflammation. Int J Colorectal Dis. 1999;14:18–28.
Arsenescu R, Arsenescu V, de Villiers WJS. TNF-α and the development of the neonatal immune system: implications for inhibitor use in pregnancy. Am J Gastroenterol. 2011;106:559–562.
González S, Rodrigo L, Martínez-Borra J, et al. TNF-α-308A promoter polymorphism is associated with enhanced TNF-α production and inflammatory activity in Crohn’s patients with fistulizing disease. Am J Gastroenterol. 2003;98:1101–1106.
Nahidi L, Leach ST, Sidler MA, Levin A, Lemberg DA, Day AS. Osteoprotegerin in pediatric Crohn’s disease and the effects of exclusive enteral nutrition. Inflamm Bowel Dis. 2011;17:516–523.
Moschen AR, Kaser A, Enrich B, et al. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 2005;54:479–487.
Vidal K, Serrant P, Schlosser B, van den Broek P, Lorget F, Donnet-Hughes A. Osteoprotegerin production by human intestinal epithelial cells: a potential regulator of mucosal immune responses. Am J Physiol Gastrointest Liver Physiol. 2004;287:G836–G844.
Gibson PR. Increased gut permeability in Crohn’s disease: is TNF the link? Gut. 2004;53:1724–1725.
Clowes JA, Riggs BL, Khosla S. The role of the immune system in the pathophysiology of osteoporosis. Immunol Rev. 2005;208:207–227.
Grattendick KJ, Nakashima JM, Feng L, Giri SN, Margolin SB. Effects of three anti-TNF-α drugs: etanercept, infliximab and pirfenidone on release of TNF-α in medium and TNF-α associated with the cell in vitro. Int J Immunopharmacol. 2008;8:679–687.
Ma TY, Boivin MA, Ye D, Pedram A, Said HM. Mechanism of TNF-{alpha} modulation of Caco-2 intestinal epithelial tight junction barrier: role of myosin light-chain kinase protein expression. Am J Physiol Gastrointest Liver Physiol. 2005;288:G422–G430.
Nahidi L, Day AS, Lemberg DA, Leach ST. Differential effects of nutritional and non-nutritional therapies on intestinal barrier function in an in vitro model. J Gastroenterol. 2012;47:107–117.
Franchimont N, Reenaers C, Lambert C, et al. Increased expression of receptor activator of NF-kappaB ligand (RANKL), its receptor RANK and its decoy receptor osteoprotegerin in the colon of Crohn’s disease patients. Clin Exp Immunol. 2004;138:491–498.
Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17- and TNF-{alpha}-induced genes in bone cells. J Leukoc Biol. 2005;77:388–399.
Sylvester FA, Draghi A, Bausero MA, Fernandez ML, Vella AT. Osteoprotegerin expression is upregulated in the colon of children with active inflammatory bowel disease. Gastroenterology. 2012;142:S209. (Abstract).
Skinner A, Lerer T, Wyzga N, Viswanathan A, Sylvester F. S1225 fecal osteoprotegerin: a marker for pediatric ulcerative colitis at diagnosis—a pilot study. Gastroenterology. 2008;134:A205.
Sylvester FA, Turner D, Draghi A II, et al. Fecal osteoprotegerin may guide the introduction of second-line therapy in hospitalized children with ulcerative colitis. Inflamm Bowel Dis. 2011;17:1726–1730.
de Jong NSH, Leach ST, Day AS. Polymeric formula has direct anti-inflammatory effects on enterocytes in an in vitro model of intestinal inflammation. Dig Dis Sci. 2007;52:2029–2036.
Feighery LM, Cochrane SW, Quinn T, et al. Myosin light chain kinase inhibition: correction of increased intestinal epithelial permeability in vitro. Pharm Res. 2008;25:1377–1386.
Belibasakis GN, Johansson A, Wang Y, Chen C, Kalfas S, Lerner UH. The cytolethal distending toxin induces receptor activator of NF-κB ligand expression in human gingival fibroblasts and periodontal ligament cells. Infect Immun. 2005;23:342–351.
Stevenson BR, Anderson JM, Bullivant S. The epithelial tight junction: structure, function and preliminary biochemical characterization. Mol Cell Biochem. 1988;83:129–145.
Zeissig S, Bojarski C, Buergel N, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–1302.
Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. 2009;1:a000034.
Nishikori M. Classcal and alternative NF-κB activation pathways and their roles in lymphoid malignancies. J Clin Exp Hematopathol. 2005;45:15–24.
Vallabhapurapu S, Karin M. Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol. 2009;27:693–733.
Tang Y, Clayburgh DR, Mittal N, et al. Epithelial NF-κB enhances transmucosal fluid movement by altering tight junction protein composition after T cell activation. Am J Pathol. 2010;176:158–167.
Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim et Biophysica Acta. 2010;1799:775–787.
Hayden MS, West AP, Ghosh S. NF-κB and the immune response. Oncogene. 2006;25:6758–6780.
Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621.
Egan LJ, Eckmann L, Greten FR, et al. IκB-kinaseβ-dependent NF-κB activation provides radioprotection to the intestinal epithelium. Proc Natl Acad Sci USA. 2004;101:2452–2457.
O’Neil DA, Porter EM, Elewaut D, et al. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724.
Kim JG, Lee SJ, Kagnoff MF. Nod1 is an essential signal transducer in intestinal epithelial cells infected with bacteria that avoid recognition by toll-like receptors. Infect Immun. 2004;73:1487–1495.
Sylvester FA, Davis PM, Wyzga N, Hyams JS, Lerer T. Are activated T cells regulators of bone metabolism in children with Crohn disease? J Pediatr. 2006;148:461–466.
Tilg H, Moschen AR, Kaser A, Pines A, Dotan I. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–694.
Miheller P, Műzes G, Rácz K, et al. Changes of OPG and RANKL concentrations in Crohn’s disease after infliximab therapy. Inflamm Bowel Dis. 2007;13:1379–1384.
Harpavat M, Greenspan SL, O’Brien C, Chang CC, Bowen A, Keljo DJ. Altered bone mass in children at diagnosis of Crohn disease: a pilot study. J Pediatr Gastroenterol Nutr. 2005;40:295–300.
Acknowledgments
This work was supported by the National Health and Medical Research Council, Australia (NHMRC, grant number 510230). Laboratory investigations were performed in the Westfield Research Laboratories.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nahidi, L., Leach, S.T., Lemberg, D.A. et al. Osteoprotegerin Exerts Its Pro-inflammatory Effects Through Nuclear Factor-κB Activation. Dig Dis Sci 58, 3144–3155 (2013). https://doi.org/10.1007/s10620-013-2851-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2851-2