Abstract
Background/Aim
We have previously reported that bee venom (BV) has a protective role against acute pancreatitis (AP). However, the effects of apamin, the major compound of BV, on AP have not been determined. The aim of this study was to evaluate the effects of apamin on cerulein-induced AP.
Methods
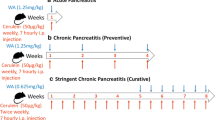
AP was induced via intraperitoneal injection of supramaximal concentrations of the stable cholecystokinin analogue cerulein (50 μg/kg) every hour for 6 times. In the apamin treatment group, apamin was administered subcutaneously (10, 50, or 100 μg/kg) at both 18 and 1 h before the first cerulein injection. The mice were sacrificed at 6 h after the final cerulein injection. Blood samples were obtained to determine serum amylase and lipase levels, as well as cytokine production. The pancreas and lung were rapidly removed for morphologic and histological examination, myeloperoxidase (MPO) assay, and real-time reverse transcription-polymerase chain reaction. Furthermore, we isolated the pancreatic acinar cells to specify the role of apamin in AP.
Results
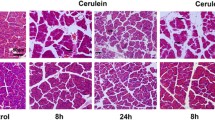
Pre-treatment with apamin inhibited histological damage, pancreatic weight/body weight ratio, serum level of amylase and lipase, MPO activity, and cytokine production. In addition, apamin treatment significantly inhibited cerulein-induced pancreatic acinar cell death. Furthermore, apamin treatment inhibited the cerulein-induced activation of c-Jun NH2-terminal kinases (JNK).
Conclusions
These results could suggest that apamin could protect against AP by inhibition of JNK activation.







Similar content being viewed by others
References
Kusske AM, Rongione AJ, Reber HA. Cytokines and acute pancreatitis. Gastroenterology. 1996;110:639–642.
Bhatia M, Wong FL, Cao Y, et al. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144.
Norman J, Franz M, Messina J, et al. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery. 1995;117:648–655.
Büchler MW, Gloor B, Müller CA, Friess H, Seiler CA, Uhl W. Acute necrotizing pancreatitis: treatment strategy according to the status of infection. Ann Surg. 2000;232:619–626.
Kwon YB, Lee JD, Lee HJ, et al. Bee venom injection into an acupuncture point reduces arthritis associated edema and nociceptive responses. Pain. 2001;90:271–280.
Billingham ME, Morley J, Hanson JM, Shipolini RA, Vernin CA. Letter: an anti-inflammatory peptide from bee venom. Nature. 1973;245:163–164.
Yun SW, Bae GS, Kim MS, et al. Melittin inhibits cerulein-induced acute pancreatitis via inhibition of the JNK pathway. Int Immunopharmacol. 2011;11:2062–2072.
Kim SJ, Park JH, Kim KH, et al. The protective effect of apamin on LPS/Fat-induced atherosclerotic mice. Evid Based Complement Alternat Med 2012:305454.
Yamauchi H, Miura M, Ichinose M, et al. Involvement of apamin-sensitive K+ channels in antigen-induced spasm of guinea-pig isolated trachea. Br J Pharmacol.. 1994;112:958–962.
Seo SW, Jung WS, Lee SE, et al. Effects of bee venom on cholecystokinin octapeptide-induced acute pancreatitis in rats. Pancreas. 2008;36:22–29.
Kallarackal AJ, Simard JM, Bailey AM. The effect of apamin, a small conductance calcium activated potassium (SK) channel blocker, on a mouse model of neurofibromatosis 1. Behav Brain Res. 2013;237:71–75.
Castle NA, Haylett DG, Jenkinson DH. Toxins in the characterization of potassium channels. Trends Neurosci. 1989;12:59–65.
Hsieh YC, Chang PC, Hsueh CH, et al. Apamin-sensitive potassium current modulates action potential duration restitution and arrhythmogenesis of failing rabbit ventricles. Circ Arrhythm Electrophysiol. 2013;6:410–418.
Dilly S, Philippart F, Lamy C, et al. The interactions of apamin and tetraethylammonium are differentially affected by single mutations in the pore mouth of small conductance calcium-activated potassium (SK) channels. Biochem Pharmacol. 2013;85:560–569.
Parajuli SP, Hristov KL, Soder RP, Kellett WF, Petkov GV. NS309 decreases rat detrusor smooth muscle membrane potential and phasic contractions by activating SK3 channels. Br J Pharmacol. 2013;168:1611–1625.
Kallarackal AJ, Simard JM, Bailey AM. The effect of apamin, a small conductance calcium activated potassium (SK) channel blocker, on a mouse model of neurofibromatosis 1. Behav Brain Res. 2013;237:71–75.
Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43.
Patel S, Ramakrishnan L, Rahman T, et al. The endo-lysosomal system as an NAADP-sensitive acidic Ca(2+) store: role for the two-pore channels. Cell Calcium. 2011;50(2):157–167.
Malinska D, Mirandola SR, Kunz WS. Mitochondrial potassium channels and reactive oxygen species. FEBS Lett. 2010;584:2043–2048.
Lee WK, Braun M, Langelüddecke C, Thévenod F. Cyclosporin a, but not FK506, induces osmotic lysis of pancreas zymogen granules, intra-acinar enzyme release, and lysosome instability by activating K+ channel. Pancreas. 2012;41:596–604.
Venglovecz V, Hegyi P, Rakonczay Z Jr, et al. Pathophysiological relevance of apical large-conductance Ca2+-activated potassium channels in pancreatic duct epithelial cells. Gut. 2011;60:361–369.
Yang K, Ding YX, Chin WC. K+-induced ion-exchanges trigger trypsin activation in pancreas acinar zymogen granules. Arch Biochem Biophys. 2007;459:256–263.
Fan M, Chambers TC. Role of MAPKs in the response of tumor cells to chemotherapy. Drug Resist Updat. 2001;4:253–267.
Gukovsky I, Gukovskaya AS, Blinman TA, Zaninovic V, Pandol SJ. Early NF-kappaB activation is associated with hormone-induced pancreatitis. Am J Physiol. 1998;275:1402–1414.
Lee J, Seo J, Kim H, Chung JB, Kim KH. Signal transduction of cerulein induced cytokine expression and apoptosis pancreatic acinar cells. Ann NY Acad Sci. 2003;1010:104–108.
Bae GS, Park HJ, Kim DY, et al. Nardostachys jatamansi protects against cerulein-induced acute pancreatitis. Pancreas. 2010;39:520–529.
Kim TH, Bae GS, Oh HJ, et al. 2′,4′,6′-Tris(methoxymethoxy) chalcone (TMMC) attenuates the severity of cerulein-induced acute pancreatitis and associated lung injury. Am J Physiol Gastrointest Liver Physiol. 2011;301:G694–G706.
Bae GS, Kim MS, Jeong J, et al. Piperine ameliorates the severity of cerulein-induced acute pancreatitis by inhibiting the activation of mitogen activated protein kinases. Biochem Biophys Res Commun. 2011;410:382–388.
Acknowledgment
This work was supported by a National Research Foundation of Korea [NRF] grant-funded by the Korean government [MEST]; Contract/Grant Number: 2010-0029498.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Gi-Sang Bae, Kwang-Ho Heo and Kyoung-Chel Park contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bae, GS., Heo, KH., Park, KC. et al. Apamin Attenuated Cerulein-Induced Acute Pancreatitis by Inhibition of JNK Pathway in Mice. Dig Dis Sci 58, 2908–2917 (2013). https://doi.org/10.1007/s10620-013-2800-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2800-0