Abstract
Background
Perifosine, an alkylphospholipid, is an Akt inhibitor which inhibits the growth of diverse cancer cells. We have reported its inhibitory effects on the growth of gastric cancer cells recently, but its molecular mechanisms are still largely unknown.
Aims
The purpose of this study was to investigate the effect and regulatory mechanism of perifosine in gastric cancer.
Methods
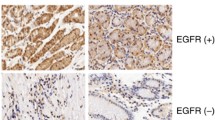
Cell viability was determined by sulforhodamine B assay after transiently transfected with AEG-1 specific siRNAs. qRT-PCR and western blot assay were used to determine the mRNA expression and proteins levels of cell signaling molecules examined. Immunohistochemistry was used to detect the AEG-1 expression in 87 gastric carcinomas, 60 dysplasia, and 47 normal gastric mucosa.
Results
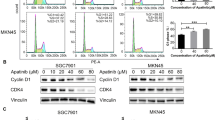
Perifosine decreased AEG-1 gene expression along with inhibition of Akt/GSK3β/C-MYC signaling pathway. Knockdown of AEG-1 using siRNA led to significant down-regulation of cyclin D1 expression at both mRNA level and protein level, and inhibited the growth of gastric cancer cells. AEG-1 expression was elevated in gastric dysplasia and cancer tissues compared to normal gastric mucosa (P < 0.01). AEG-1 over-expression correlated with diffuse type of gastric cancer and advanced tumor stages.
Conclusions
Perifosine inhibits the growth of gastric cancer cells possibly through inhibition of the Akt/GSK3β/C-MYC signaling pathway—mediated down-regulation of AEG-1 that subsequently down-regulated cyclin D1. AEG-1 may play an important role in the carcinogenesis and progression of gastric cancer and could be a therapeutic target of perifosine.





Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Milne AN, Carneiro F, O’Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–628.
Orditura M, De Vita F, Muto P, et al. Adjuvant chemoradiotherapy in patients with stage III or IV radically resected gastric cancer: a pilot study. Arch Surg. 2010;145:233–238.
Hideshima T, Catley L, Yasui H, et al. Perifosine, an oral bioactive novel alkylphospholipid, inhibits Akt and induces in vitro and in vivo cytotoxicity in human multiple myeloma cells. Blood. 2006;107:4053–4062.
Elrod HA, Lin YD, Yue P, et al. The alkylphospholipid perifosine induces apoptosis of human lung cancer cells requiring inhibition of Akt and activation of the extrinsic apoptotic pathway. Mol Cancer Ther. 2007;6:2029–2038.
Papa V, Tazzari PL, Chiarini F. Proapoptotic activity and chemosensitizing effect of the novel Akt inhibitor perifosine in acute myelogenous leukemia cells. Leukemia. 2008;22:147–160.
Tazzari PL, Tabellini G, Ricci F, et al. Synergistic proapoptotic activity of recombinant TRAIL plus the Akt inhibitor perifosine in acute myelogenous leukemia cells. Cancer Res. 2008;68:9394–9403.
Fu L, Kim YA, Wang X, et al. Perifosine inhibits mammalian target of rapamycin signaling through facilitating degradation of major components in the mTOR axis and induces autophagy. Cancer Res. 2009;69:8967–8976.
Rahmani M, Reese E, Dai Y, et al. Coadministration of histone deacetylase inhibitors and perifosine synergistically induces apoptosis in human leukemia cells through Akt and ERK1/2 inactivation and the generation of ceramide and reactive oxygen species. Cancer Res. 2005;65:2422–2432.
Nyakern M, Cappellini A, Mantovani I, Martelli AM. Synergistic induction of apoptosis in human leukemia T cells by the Akt inhibitor perifosine and etoposide through activation of intrinsic and Fas-mediated extrinsic cell death pathways. Mol Cancer Ther. 2006;5:1559–1570.
Dasmahapatra GP, Didolkar P, Alley MC, Ghosh S, Sausville EA, Roy KK. In vitro combination treatment with perifosine and UCN-01 demonstrates synergism against prostate (PC-3) and lung (A549) epithelial adenocarcinoma cell lines. Clin Cancer Res. 2004;10:5242–5252.
Festuccia C, Gravina GL, Muzi P, et al. Akt down-modulation induces apoptosis of human prostate cancer cells and synergizes with EGFR tyrosine kinase inhibitors. Prostate. 2008;68:965–974.
Su ZZ, Kang DC, Chen Y, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602.
Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte elevated gene-1 (AEG-1) is a target gene of oncogenic Ha-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci USA. 2006;103:17390–17395.
Wang X, Yue P, Kim YA, Fu H, Khuri FR, Sun SY. Enhancing mammalian target of rapamycin (mTOR)-targeted cancer therapy by preventing mTOR/raptor inhibition-initiated, mTOR/rictor-independent Akt activation. Cancer Res. 2008;68:7409–7418.
Luo X, Fan S, Huang W, et al. Downregulation of IRS-1 promotes metastasis of head and neck squamous cell carcinoma. Oncol Rep. 2012;28:659–667.
Hirotsu M, Setoguchi T, Sasaki H, et al. Smoothened as a new therapeutic target for human osteosarcoma. Mol Cancer. 2010;9:5.
Dubrovska A, Kim S, Salamone RJ, et al. The role of PTEN/Akt/PI3 K signaling in the maintenance and viability of prostate cancer stem-like cell populations. Proc Natl Acad Sci USA. 2009;106:268–273.
Liu D, Sun Q, Liang S et al. MicroRNA-27a inhibitors alone or in combination with perifosine suppress the growth of gastric cancer cells. Mol Med Rep. 2013;7:642–648.
Liang S, Guo R, Zhang Z, et al. Upregulation of the eIF4E signaling pathway contributes to the progression of gastric cancer, and targeting eIF4E by perifosine inhibits cell growth. Oncol Rep. 2013;29:2422–2430.
Gills JJ, Dennis PA. Perifosine: update on a novel Akt inhibitor. Curr Oncol Rep. 2009;11:102–110.
Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK, et al. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093–1103.
Lee SG, Su ZZ, Emdad L, Sarkar D, Franke TF, Fisher PB. Astrocyte elevated gene-1 activates cell survival pathways through PI3 K-Akt signaling. Oncogene. 2008;27:1114–1121.
Mishra R. Glycogen synthase kinase 3 beta: can it be a target for oral cancer. Mol Cancer. 2010;9:144.
Jian-bo X, Hui W, Yu-long H, et al. Astrocyte-elevated gene-1 overexpression is associated with poor prognosis in gastric cancer. Med Oncol. 2011;28:455–462.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 30873099, 81102458, 81172004) (X. Wang), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (X. Wang), the Nanjing Pharmaceutical Technology Development Project (YKK09050) (W. Huang), “Medical ZhongDianRenCai Project” of Jiangsu Province (RC2011059) (L. Yang), and ‘‘Six RenCai Gaofeng’’ Funding for the Young Academic Leader of Jiangsu Province (2012) (L. Yang). We thank Dr. Heath Elrod for editing of the manuscript.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Wenbin Huang and Li Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Huang, W., Yang, L., Liang, S. et al. AEG-1 Is a Target of Perifosine and Is Over-Expressed in Gastric Dysplasia and Cancers. Dig Dis Sci 58, 2873–2880 (2013). https://doi.org/10.1007/s10620-013-2735-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2735-5