Abstract
Background
It is widely accepted that the adenoma-carcinoma sequence represents the process by which most colorectal cancers (CRCs) arise. Although gankyrin is overexpressed in CRC tissues, its roles in the initiation step of colorectal carcinogenesis remain largely unexplored.
Aim
We investigated the expression of gankyrin and stemness factors in human colorectal adenomas, precancerous lesions, as well as CRC tissues to assess its involvement in colorectal carcinogenesis.
Methods
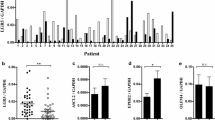
Expression of several molecules including gankyrin and certain stemness factors was compared in 50 pairs of adenoma and surrounding normal mucosa using real-time quantitative polymerase chain reaction and in 30 CRC tissues using immunohistochemistry.
Results
In CRC specimens, expression of CD133, a cancer stem cell marker, was significantly correlated with gankyrin expression. Gankyrin knockdown decreased the expression of vascular endothelial growth factor (VEGF) and stemness factors such as Nanog and Oct-4 in colorectal cancer cells. Expression of gankyrin and these stemness factors was significantly higher in adenomas than in the surrounding normal mucosa. Importantly, a significant correlation was observed between the expression of gankyrin, VEGF, and Nanog in colorectal adenomas.
Conclusion
In CRC development, gankyrin would control stem cell behavior by regulating the expression of stemness factors.




Similar content being viewed by others
Abbreviations
- CRC:
-
Colorectal cancer
- VEGF:
-
Vascular endothelial growth factor
- LST:
-
Laterally spreading tumor
References
Ferlay J, Shin HR, Bray F et al. Cancer incidence and mortality worldwide. GLOBOCAN 2008 v1.2. IARC CancerBase No. 10; 2008.
Kudo S. Endoscopic mucosal resection of flat and depressed types of early colorectal cancer. Endoscopy. 1993;25:455–461.
Teixeira CR, Tanaka S, Haruma K, et al. Flat-elevated colorectal neoplasms exhibit a high malignant potential. Oncology. 1996;53:89–93.
Saitoh Y, Waxman I, West AB, et al. Prevalence and distinctive biologic features of flat colorectal adenomas in a North American population. Gastroenterology. 2001;120:1657–1665.
Hurlstone DP, Korulla C, Lobo AJ. Colorectal laterally spreading tumors: clinical evaluation and endoscopic strategies updated. J Gastroenterol Hepatol. 2002;17:1344–1345.
Kudo S, Kashida H, Nakajima T, et al. Endoscopic diagnosis and treatment of early colorectal cancer. World J Surg. 1997;21:694–701.
Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111.
Clarke MF, Dick JE, Dirks PB, et al. Cancer stem cells–perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006;66:9339–9344.
O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110.
Hoei-Hansen CE. Application of stem cell markers in search for neoplastic germ cells in dysgenetic gonads, extragonadal tumours, and in semen of infertile men. Cancer Treat Rev. 2008;3:348–367.
Shan J, Shen J, Liu L, et al. Nanog regulates self-renewal of cancer stem cell through IGF pathway in human hepatocellular carcinoma. Hepatology. 2012;56:1004–1014.
Meng HM, Zheng P, Wang XY, et al. Overexpression of nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9:295–302.
Higashitsuji H, Itoh K, Nagao T, et al. Reduced stability of retinoblastoma protein by gankyrin, an oncogenic ankyrin-repeat protein overexpressed in hepatomas. Nat Med. 2000;6:96–99.
Tang S, Yang G, Meng Y, et al. Overexpression of a novel gene gankyrin correlates with the malignant phenotype of colorectal cancer. Cancer Biol Ther. 2010;9:88–95.
Higashitsuji H, Higashitsuji H, Itoh K, et al. The oncoprotein gankyrin binds to MDM2/HDM2, enhancing ubiquitylation and degradation of p53. Cancer Cell. 2005;8:75–87.
Fu J, Chen Y, Cao J, et al. p28GANK overexpression accelerates hepatocellular carcinoma invasiveness and metastasis via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1α pathways. Hepatology. 2011;53:181–192.
Sakurai T, He G, Matsuzawa A, et al. Hepatocyte necrosis induced by oxidative stress and IL-1 alpha release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer Cell. 2008;14:156–165.
Savagner P. The epithelial-mesenchymal transition (EMT) phenomenon. Ann Oncol. 2010;21:vii89–vii92.
Noro A, Sugai T, Hababo W, et al. Analysis of K-ras and p53 gene mutations in laterally spreading tumors of the colorectum. Pathol Int. 2003;53:828–836.
Beck B, Driessens G, Goossens S, et al. A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature. 2011;478:399–403.
Dong LW, Yang GZ, Pan YF, et al. The oncoprotein p28GANK establishes a positive feedback loop in β-catenin signaling. Cell Res. 2011;21:1248–1261.
Man JH, Liang B, Gu YX, et al. Gankyrin plays an essential role in Ras-induced tumorigenesis through regulation of the RhoA/ROCK pathway in mammalian cells. J Clin Invest. 2010;120:2829–2841.
Rotondano G, Bianco MA, Buffoli F, et al. The cooperative Italian FLIN study group: prevalence and clinico-pathological features of colorectal laterally spreading tumors. Endoscopy. 2011;43:856–861.
Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;45:141–146.
Chan EM, Ratanasirintrawoot S, Park IH, et al. Live cell imaging distinguishes bona fide human iPS cells from partially reprogrammed cells. Nat Biotechnol. 2009;27:1033–1037.
Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676.
Yu J, Vodyanik MA, Smuga-Otto K, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920.
Müller FJ, Laurent LC, Kostka D, et al. Regulatory networks define phenotypic classes of human stem cell lines. Nature. 2008;455:401–405.
Ferrara N. VEGF-A: a critical regulator of blood vessel growth. Eur Cytokine Netw. 2009;20:158–163.
Sakurai T, Kudo M. Signaling pathways governing tumor angiogenesis. Oncology. 2011;81:24–29.
Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732.
Sun W, Ding J, Wu K, et al. Gankyrin-mediated dedifferentiation facilitates the tumorigenicity of rat hepatocytes and hepatoma cells. Hepatology. 2011;54:1259–1272.
Qian YW, Chen Y, Yang W, et al. p28(GANK) prevents degradation of Oct4 and promotes expansion of tumor-initiating cells in hepatocarcinogenesis. Gastroenterology. 2012;142:1547–1558.
Umemura A, Itoh Y, Itoh K, et al. Association of gankyrin protein expression with early clinical stages and insulin-like growth factor-binding protein 5 expression in human hepatocellular carcinoma. Hepatology. 2008;47:493–502.
Acknowledgments
We thank Dr. Yoshie O and Munakata H (Kinki University) for technical assistance and helpful suggestions. This research was supported by grants from the Yasuda Medical Foundation, Novartis Foundation and Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mine, H., Sakurai, T., Kashida, H. et al. Association of Gankyrin and Stemness Factor Expression in Human Colorectal Cancer. Dig Dis Sci 58, 2337–2344 (2013). https://doi.org/10.1007/s10620-013-2627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-013-2627-8