Abstract
Background
One of the problems associated with infliximab (IFX) treatment for Crohn’s disease (CD) is loss of response during maintenance therapy.
Aims
The aim of this multicenter, retrospective, cohort study was to determine whether enteral nutrition (EN) added to the IFX therapy regimen is effective for maintaining remission in adult CD patients.
Methods
Patients with CD who had started IFX therapy between April 2003 and March 2008 at any one of the seven participating medical centers and who met the following inclusion criteria were enrolled in the study: remission after triple infusions of IFX followed by IFX maintenance therapy every 8 weeks, and follow-up data available for ≥1 year. Remission was defined as a C-reactive protein (CRP) level of <0.3 mg/dL, and recurrence was defined as an increase in CRP to ≥1.5 mg/dL or shortening of the IFX interval. Patients were classified by EN dosage into two groups (EN group and non-EN group). The cumulative remission period and related factors were analyzed.
Results
Of the 102 adult CD patients who met the inclusion criteria, 45 were in the EN group and 57 were in the non-EN group. The cumulative remission rate was significantly higher in the EN group than in the non-EN group (P = 0.009). Multivariate analysis revealed that EN was the only suppressive factor for disease recurrence (P = 0.01).
Conclusions
The results demonstrate that among this CD patient cohort, EN combined with IFX maintenance treatment was clinically useful for maintaining remission.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Crohn’s disease (CD) is a chronic inflammatory intestinal disease that is characterized by a disabling course. To date, there is no curative treatment for CD. However, treatment options have become more diverse in recent years, as evidenced by the clinical application of anti-cytokine therapy, including tumor necrosis factor alpha (TNF-α) inhibitor [1]. Anti-TNF-α antibodies, such as infliximab (IFX) and adalimumab, are useful for inducing CD remission and are the most effective medical treatments currently available. These drugs are also used to maintain remission, and improvement of long-term clinical outcomes of CD has been demonstrated [2, 3]. However, it is also well known that some patients do not respond to IFX treatment or produce antibodies against IFX, which decreases IFX efficacy [4, 5]. For these IFX-resistant cases, clinicians must shorten the interval of IFX administration, increase the dose, or replace IFX with adalimumab. The concomitant use of immunomodulators (IMMs) is considered to be an effective option for increasing the therapeutic effect of IFX [6], but serious complications, such as hepatosplenic T cell lymphoma, have been reported with IFX and IMM combination therapy [7]. In contrast to IMMs, enteral nutrition (EN) has a highly favorable safety profile, and a number of studies have demonstrated that EN can improve CD through mechanisms that differ from those of other therapies [8–11]. Although EN is often used for pediatric CD patients [12, 13], randomized, controlled trials have demonstrated that EN is also effective as maintenance therapy in adult CD patients [14, 15]. Therefore, we conducted a retrospective study to determine whether the addition of EN improves the maintenance of remission induced by IFX.
Materials and Methods
Seven medical institutions in Japan (Fukuoka University Chikushi Hospital, Kyushu University Hospital, Fukuoka University Hospital, Miyazaki University Hospital, Nagasaki University Hospital, Takano Hospital, and Imamura Hospital) participated in this multicenter cohort study.
Adult CD patients who were diagnosed at these hospitals and had started IFX therapy between April 2003 and March 2008 and who met the following inclusion criteria were enrolled: (1) remission after treatment with 5 mg/kg IFX at weeks 0, 2, and 6, followed by IFX maintenance therapy every 8 weeks; (2) medical records that allowed retrospective evaluation of subjective symptoms, drug use, and laboratory data for a follow-up period of at least 1 year. Since this was a retrospective study, it was not possible to calculate the CD activity index (CDAI) for all subjects. Therefore, the definition of remission was based on the results of C-reactive protein (CRP) evaluation. All patients underwent blood tests, including CRP, before the start of IFX treatment, at the start of treatment, and after the administration of IFX at weeks 2, 6, and 14. Patients were considered to have achieved remission if they had a CRP level of <0.3 mg/dL [16] between 6 and 14 weeks after treatment initiation. Recurrence was evaluated based on the patient’s medical records and CRP values. The definition of recurrence was CD-related hospitalization, additional treatment, an IFX administration interval shortened to <4 weeks, and recurrence of increased blood CRP level (to ≥1.5 mg/dL). The shortened IFX administration interval was set at ≤4 weeks because it was administered after 6 weeks in three patients who did not have a “loss of response” for patient-related reasons.
Takagi et al. [14] reported that a “half elemental diet (ED)” was useful as maintenance therapy for CD patients, and the dosage for the half ED group per day in their study was 900–1,200 kcal/day. Therefore, in our study we defined patients who had received >900 kcal/day EN as the EN group, and those who had received <900 kcal/day EN or no EN at all as the non-EN group. In Japan, EN corresponds to a healthcare act, and a prescription given by a physician is required for treatment. The doses of EN in this study were investigated and confirmed retrospectively from prescriptions in patients’ medical records, but we did not consider the type of EN or the administration route (oral or nasal). Patients’ characteristics and the cumulative remission rates were compared between the two groups, and the risk factors for recurrence were evaluated using multivariate analysis.
Statistical Analysis
Statistical analysis was performed using JMP ver. 8 (SAS Institute, Cary, NC). Patients’ characteristics were compared using the chi-square, Fisher’s exact, and Student’s t tests. The cumulative remission rate was estimated using the Kaplan–Meier method and compared using the log-rank test. Risk factors for recurrence were evaluated by multivariate analysis using a Cox proportional hazards model. In all statistical analyses, the significance level was set at 0.05.
Results
Patient Groups
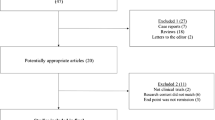
The medical records of 133 CD patients who had undergone IFX maintenance therapy were reviewed. Twenty-one patients who did not fulfill the remission criteria (CRP <0.3 mg/dL after IFX triple infusion) were excluded from the study (non-responders). An additional seven patients were excluded due to insufficient follow-up periods, and three were excluded because of an atypical IFX administration schedule in weeks 0, 2, 6, and 14. Ultimately, 102 CD patients were included in the analysis. Because this was a retrospective study, physicians at each institution decided upon the kind of combination therapy with IFX without a confirmed rule. Up until the present time, EN has been widely used as maintenance therapy for CD patients in Japan. As a result, 45 of 102 patients (44 %) were in the EN group, and 57 (56 %) were in the non-EN group. The mean EN intake in the EN group was 1,233 ± 62 kcal/day. Of the 57 patients in the non-EN group, 24 ingested <900 kcal/day, with a mean intake of 535 ± 32 kcal/day. The prescribed enteral supplement was Elental in 63 % of patients; the other 37 % had a semi-ED or low residual diet.
Patients’ Characteristics
Of the 102 patients, 78 (75 %) were male and 28 (27.5 %) were smokers. Table 1 shows the characteristics of the two groups. Patients were significantly older in the EN group than in the non-EN group (P = 0.03). The frequency of perianal lesions was not significantly different between the two groups, and perianal abscess was seen in only two patients in the EN group and two patients in the non-EN group. The incidence of intestinal stenosis was also significantly higher in the EN group (P = 0.05). There was no significant difference in the percentage of patients who received prednisolone or azathioprine. No other characteristics differed significantly between the two groups.
Cumulative Remission Rate
The average follow-up period was 544.1 ± 26.5 days and did not differ significantly between the two groups (525.3 ± 45.1 vs. 558.9 ± 31.3 days for the EN vs. non-EN group, respectively). The cumulative remission rate was significantly higher in the EN group than in the non-EN group (Fig. 1). Of the 45 patients in the EN group, 14 (31.1 %) had recurrence during the follow-up period. The determination of recurrence in these patients was based on increased CRP level (11 patients) and shortened IFX administration interval (3 patients). In comparison, 33 of the 57 patients (57.8 %) in the non-EN group had recurrence based on increased CRP level (28 patients) and shortened IFX administration interval (5 patients).
Based on these results, risk factors for recurrence were evaluated by multivariate analysis. This analysis was performed for various characteristics, disease location, disease behavior, and concomitant drugs using the Cox proportional hazards model. The only independent significant risk factor for recurrence was the intake of >900 kcal/day EN. The multivariate hazard ratio (HR) was 0.423, with a 95 % confidence interval (CI) of 0.21–0.83 (Table 2).
Two patients in the EN group (4.4 %) and two patients in the non-EN group (3.5 %) required surgery, with no significant difference between these two groups (P = 0.58).
Safety Profile
Adverse reactions were observed in eight patients during IFX maintenance therapy; five reported mild infusion reactions (2 in the EN group and 3 in the non-EN group), and three had infection (all in the non-EN group). All patients improved after conservative therapy. There were no serious adverse reactions that required hospitalization or discontinuation of IFX treatment.
Discussion
Crohn’s disease is associated with recurrences and remissions, and patients often require intestinal resection due to complications, such as intestinal stenosis and fistula formation, during the long-term course of the disease [17, 18]. Quality of life is compromised in many patients due to frequent intestinal resection or long-term concomitant prednisolone therapy. However, the advent of new treatment methods, especially anti-cytokine therapy, such as TNF-α inhibitors, has greatly changed CD treatment options. Even patients who are resistant to conventional treatment can show a higher remission rate after anti-cytokine therapy, which is also effective for the maintenance of remission [1–3]. In Japan, IFX for CD treatment was introduced in 2002 and has been widely used since then. However, loss of efficacy is observed in some patients during maintenance therapy after they have responded to treatment and achieved remission [4, 5]. This is the well-known phenomenon of loss of response and is considered to be mainly due to decreased blood drug concentrations caused by the presence of anti-IFX antibodies [19]. In a review of 16 studies, loss of response to infliximab was seen in 37 % of all CD patients [20]; the follow-up period in each study differed, but when the length of time is taken into account, loss of response was 13 % per patient-year [20]. For these patients with a loss of response to IFX, treatment options include shortening the interval of IFX administration, increasing the dose, or replacing IFX with adalimumab. However, patients often still do not respond sufficiently even after such measures are taken. This has led to the use of combination therapies with IFX aimed at preventing loss of response to the drug. Among such combination therapies, IMMs are reported to be the most effective [6]. However, the risks and benefits of combination therapy with IFX and IMMs remain controversial, and no consensus has been yet reached because severe complications, such as hepatosplenic T cell lymphoma, have been reported [7].
EN is effective for the treatment of CD and has been used as a first-line treatment for remission induction in pediatric CD [12, 13]. EN is also able to maintain remission in adult CD patients [21]. Randomized, controlled trials have demonstrated that an ED consisting of half the necessary calories effectively maintains remission [14]. EN prevents not only active naïve lesions of CD, but also postoperative recurrence of the disease [22]. Tanaka et al. reported that concomitant EN therapy is also effective after triple infusions of IFX [23]. The preventive effect is considered to be associated with the anti-inflammatory effect [8], activity related to mucosal cytokines [9], correction of gut permeability [10], and modification of gut flora [11]. In our study, we focused on the maintenance of remission with EN and assessed its usefulness, especially when used in combination with IFX. To exclude those patients who did not respond to the initial treatment, 102 CD patients who were confirmed to have achieved remission were followed up using data retrieved from their medical records. With an average follow-up period of 544.1 ± 26.5 days, the cumulative remission rate was significantly higher in the EN group than in the non-EN group (P = 0.009). Multivariate analysis showed that the use of EN was the only factor that was significantly associated with disease recurrence. These findings suggest that EN combined with IFX improves the remission effect of IFX. EN has a favorable safety profile due to its non-pharmaceutical properties, suggesting that it might be a good alternative to IMMs in IFX combination therapy. However, in order to continue EN therapy at the necessary dose for a long time, it is important to address issues regarding treatment compliance. Nutrients used for an ED have a particular amino acid taste, which makes it difficult for some patients to take large amounts of EN, leading to poor compliance. Several issues, including the taste of EN, should be resolved to facilitate long-term EN treatment for the maintenance of CD remission.
This study had several limitations related to its retrospective nature. First, the results may have been biased because there were differences in the patients’ characteristics between the EN and non-EN groups. Intestinal stenosis was significantly more common in the EN group than in the non-EN group. Symptoms of intestinal stenosis were to some degree reduced with EN. In the EN group, the amount of dietary intake decreased because of symptoms of stenosis, as a result of which it was possible to increase the amount of EN. Instances of high-level symptoms of stenosis were seen in some patients when CD was not active, but generally intestinal stenosis often co-exists with advanced lesions and is thought to be a risk factor for recurrence. Therefore, the presence of intestinal stenosis did not decrease recurrence in the EN group and was unlikely to have exerted a major effect on the results of our investigation. In addition, while the difference was not significant, there were more smokers in the non-EN group than in the EN group. In addition, smoking is known to have a negative effect on the course of CD [24, 25]. This was not a large study, and smoking or non-smoking could have had some effect on the results. Furthermore, remission and recurrence could not be defined using the CDAI, which is generally used to measure disease activity. In particular, because we defined remission according to the CRP level, no subjective and objective manifestations were reflected. The results might have been different if remission and recurrence were defined using the CDAI. However, other studies have suggested that CRP correlates well with clinical activity and with endoscopic and histological findings [26] and that simple determination based on the CRP level and the erythrocyte sedimentation rate can be used as a short-term prognostic index [27]. In a cohort study, Schnitzler et al. used methods similar to those used in our study to evaluate biological activity [16]. Also, evidence of a good correlation between responsiveness to IFX and CRP was reported in recent large-scale trials [28, 29]. There is room for discussion about whether CRP accurately reflects activity to the same degree as CDAI. However, in our retrospective study, recurrence was defined using CRP, which could be evaluated, and the need for further hospitalization, additional treatment, and shortened IFX infusion interval were added as additional criteria. Thus, clinically, the definition was quite strict.
Some CD patients cannot be managed by IFX or other TNF-α inhibitor therapy alone. EN can be used safely and improves the therapeutic effect of IFX through a mechanism different from that of IMMs. The results of our study confirm that EN in combination with IFX therapy is effective for remission maintenance. Yamamoto et al. [30] conducted a single-center prospective study to investigate the usefulness of combined EN and IFX maintenance therapy and found no such benefit of EN. The sample size of their study was small, and the follow-up period was also short compared to our study, which may account for the different results. To determine the usefulness of EN in combination with IFX maintenance therapy, a large-scale, multicenter, randomized, controlled trial is necessary.
References
Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med. 1997;337:1029–1035.
Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet. 2002;359:1541–1549.
Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med. 2004;350:876–885.
Rutgeerts P, Van Assche G, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593–1610.
Gisbert JP, Panes J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767.
Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. 2010;15:1383–1395.
Mackey AC, Green L, Leptak C, et al. Hepatosplenic T cell lymphoma associated with infliximab use in young patients treated for inflammatory bowel disease: update. J Pediatr Gastroenterol Nutr. 2009;48:386–388.
Fell JM, Paintin M, Arnaud-Battandier F, et al. Mucosal healing and a fall in mucosal pro-inflammatory cytokine mRNA induced by a specific oral polymeric diet in paediatric Crohn’s disease. Aliment Pharmacol Ther. 2000;14:281–289.
Yamamoto T, Nakahigashi M, Saniabadi AR, et al. Impacts of long-term enteral nutrition on clinical and endoscopic disease activities and mucosal cytokines during remission in patients with Crohn’s disease: a prospective study. Inflamm Bowel Dis.. 2007;13:1493–1501.
Guzy C, Schirbel A, Paclik D, et al. Enteral and parenteral nutrition distinctively modulate intestinal permeability and T cell function in vitro. Eur J Nutr. 2009;48:12–21.
Learch ST, Mitchell HM, Eng WR, et al. Sustained modulation of intestinal bacteria by exclusive enteral nutrition used to treat children with Crohn’s disease. Aliment Pharmacol Ther. 2008;28:724–733.
Travis SP, Stange EF, Lemann M, et al. European evidence based consensus on the diagnosis and management of Crohn’s disease: current management. Gut. 2006;55:i16–35.
Berni Canani R, Terrin G, Borrelli O, et al. Short- and long-term therapeutic efficacy of nutritional therapy and corticosteroids in paediatric Crohn’s disease. Dig Liver Dis. 2006;38:381–387.
Takagi S, Utsunomiya K, Kuriyama S, et al. Effectiveness of an ‘half elemental diet’ as maintenance therapy for Crohn’s disease: a randomized-controlled trial. Aliment Pharmacol Ther. 2006;24:1333–1340.
Takagi S, Utsunomiya K, Kuriyama S, et al. Quality of life of patients and medical cost of “half elemental diet” as maintenance therapy for Crohn’s disease: secondary outcomes of a randomised controlled trial. Dig Liver Dis. 2009;41:390–394.
Schnitzler F, Fidder H, Ferrante M, et al. Long-term outcome of treatment with infliximab in 614 patients with Crohn’s disease: results from a single-centre cohort. Gut. 2009;58:492–500.
Cosnes J, Cattan S, Blain A, et al. Long-term evolution of disease behavior of Crohn’s disease. Inflamm Bowel Dis. 2002;8:244–250.
Greenstein AJ, Lachman P, Sachar DB, et al. Perforating and non-perforating indications for repeated operations in Crohn’s disease: evidence for two clinical forms. Gut. 1988;29:588–592.
Maser EA, Villela R, Silverberg MS, et al. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol. 2006;4:1248–1254.
Gisbert JP, Panés J. Loss of response and requirement of infliximab dose intensification in Crohn’s disease: a review. Am J Gastroenterol. 2009;104:760–767.
Verma S, Kirkwood B, Brown S, et al. Oral nutritional supplementation is effective in the maintenance of remission in Crohn’s disease. Dig Liver Dis. 2000;32:769–774.
Esaki M, Matsumoto T, Hizawa K, et al. Preventive effect of nutritional therapy against postoperative recurrence of Crohn’s disease, with reference to findings determined by intra-operative enteroscopy. Scand J Gastroenterol. 2005;40:1431–1437.
Tanaka T, Takahama K, Kimura T, et al. Effect of concurrent elemental diet on infliximab treatment for Crohn’s disease. J Gastroenterol Hepatol.. 2006;21:1143–1149.
Song XM, Gao X, Li MZ, et al. Clinical features and risk factors for primary surgery in 205 patients with Crohn’s disease: analysis of a South China cohort. Dis Colon Rectum. 2011;54:1147–1154.
Seksik P, Nion-Larmurier I, Sokol H, et al. Effects of light smoking consumption on the clinical course of Crohn’s disease. Inflamm Bowel Dis. 2009;15:734–741.
Solem CA, Loftus EV Jr, Tremaine WJ, et al. Correlation of C-reactive protein with clinical, endoscopic, histologic, and radiographic activity in inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:707–712.
Consigny Y, Modigliani R, Colombel JF, et al. A simple biological score for predicting low risk of short-term relapse in Crohn’s disease. Inflamm Bowel Dis. 2006;12:551–557.
Jürgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol.. 2011;9:421–427.
Reinisch W, Wang Y, Oddens BJ, et al. C-reactive protein, an indicator for maintained response or remission to infliximab in patients with Crohn’s disease: a post hoc analysis from ACCENT I. Aliment Pharmacol Ther. 2012;35:568–576.
Yamamoto T, Nakahigashi M, Umegae S, et al. Prospective clinical trial: enteral nutrition during maintenance infliximab in Crohn’s disease. J Gastroenterol. 2010;45:24–29.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Hirai, F., Ishihara, H., Yada, S. et al. Effectiveness of Concomitant Enteral Nutrition Therapy and Infliximab for Maintenance Treatment of Crohn’s Disease in Adults. Dig Dis Sci 58, 1329–1334 (2013). https://doi.org/10.1007/s10620-012-2374-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2374-2