Abstract
Background
Protein products of klothoβ (KLB) and fibroblast growth factor receptor 4 (FGFR4) impact fibroblast growth factor 19-mediated feedback inhibition of hepatic bile acid (BA) synthesis. Variants of KLB and FGFR4 influence colonic transit (CT) in diarrhea-predominant irritable bowel syndrome (IBS-D).
Aim
The purpose of this study was to test the hypothesis that colesevelam’s slowing effects on CT in IBS-D patients is influenced by genetic variants in KLB and FGFR4.
Methods
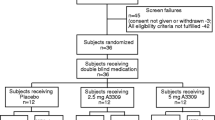
We examined pharmacogenetic effects of KLB and FGFR4 coding variants (SNPs) on scintigraphic CT response to the BA sequestrant, colesevelam 1.875 g b.i.d. versus placebo (PLA) for 14 days in 24 female IBS-D patients.
Results
FGFR4 rs351855 and KLB rs497501 were associated with differential colesevelam effects on ascending colon (AC) half-emptying time (t 1/2, P = 0.046 and P = 0.085 respectively) and on overall CT at 24 h (geometric center, GC24: P = 0.073 and P = 0.042, respectively), with slower transit for rs351855 GA/AA (but not for GG) and rs497501 CA/AA (but not CC) genotypes.
Conclusion
FGFR4 rs351855 and KLB rs4975017 SNPs may identify a subset of IBS-D patients with beneficial response to colesevelam.




Similar content being viewed by others
References
Hofmann AF, Mangelsdorf DJ, Kliewer SA. Chronic diarrhea due to excessive bile acid synthesis and not defective ileal transport: a new syndrome of defective fibroblast growth factor 19 release. Clin Gastroenterol Hepatol. 2009;7:1151–1154.
Walters JR, Tasleem AM, Omer OS, Brydon WG, Dew T, le Roux CW. A new mechanism for bile acid diarrhea: defective feedback inhibition of bile acid biosynthesis. Clin Gastroenterol Hepatol. 2009;7:1189–1194.
Wedlake L, A’Hern R, Russell D, Thomas K, Walters JR, Andreyev HJ. Systematic review: the prevalence of idiopathic bile acid malabsorption as diagnosed by SeHCAT scanning in patients with diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther. 2009;30:707–717.
Nyhlin H, Merrick MV, Eastwood MA, Brydon WG. Evaluation of ileal function using 23-selena-25-homotaurocholate, a-gamma-labeled conjugated bile acid. Initial clinical assessment. Gastroenterology. 1983;84:63–68.
Odunsi-Shiyanbade ST, Camilleri M, McKinzie S, et al. Effects of chenodeoxycholate and a bile acid sequestrant, colesevelam, on intestinal transit and bowel function. Clin Gastroenterol Hepatol. 2010;8:159–165.
Brydon WG, Nyhlin H, Eastwood MA, Merrick MV. Serum 7 alpha-hydroxy-4-cholesten-3-one and selenohomocholyltaurine (SeHCAT) whole body retention in the assessment of bile acid induced diarrhoea. Eur J Gastroenterol Hepatol. 1996;8:117–123.
Wong BS, Camilleri M, Carlson PJ, et al. A klothoβ variant mediates protein stability and associates with colon transit in irritable bowel syndrome with diarrhea. Gastroenterology. 2011;140:1934–1942.
Rao AS, Wong BS, Camilleri M, et al. Chenodeoxycholate in females with irritable bowel syndrome-constipation: a pharmacodynamic and pharmacogenetic analysis. Gastroenterology. 2010;139:1549–1558.
Camilleri M. Scintigraphic biomarkers for colonic dysmotility. Clin Pharmacol Ther. 2010;87:748–753.
Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423.
Viramontes BE, Camilleri M, McKinzie S, Pardi DS, Burton D, Thomforde GM. Gender related differences in slowing colonic transit by a 5-HT3 antagonist in subjects with diarrhea-predominant irritable bowel syndrome. Am J Gastroenterol. 2001;92:2671–2676.
Camilleri M, Nadeau A, Tremaine WJ, et al. Measurement of serum 7alpha-hydroxy-4-cholesten-3-one (or 7alphaC4), a surrogate test for bile acid malabsorption in health, ileal disease and irritable bowel syndrome using liquid chromatography-tandem mass spectrometry. Neurogastroenterol Motil. 2009;21:734–e43.
Spiller R, Camilleri M, Longstreth GF. Perspective: do the symptom-based, Rome criteria of irritable bowel syndrome lead to better diagnosis and treatment outcomes? The con argument. Clin Gastroenterol Hepatol. 2010;8:125–136.
Sanders DS, Carter MJ, Hurlstone DP, et al. Association of adult coeliac disease with irritable bowel syndrome: a case-control study in patients fulfilling ROME II criteria referred to secondary care. Lancet. 2001;358:1504–1508.
El-Salhy M, Lomholt-Beck B, Gundersen D. The prevalence of celiac disease in patients with irritable bowel syndrome. Mol Med Rep. 2011;4:403–405.
Korkut E, Bektas M, Oztas E, Kurt M, Cetinkaya H, Ozden A. The prevalence of celiac disease in patients fulfilling Rome III criteria for irritable bowel syndrome. Eur J Intern Med. 2010;21:389–392.
Acknowledgments
We thank Mary Lempke, Pharm. D., research pharmacist, and Cindy Stanislav, secretary, for assistance. This work is funded by grant RO1-DK092179 from the National Institutes of Health to Dr. Camilleri and by Mayo Clinic CTSA grant (RR24150).
Conflict of interest
The authors have no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wong, B.S., Camilleri, M., Carlson, P.J. et al. Pharmacogenetics of the Effects of Colesevelam on Colonic Transit in Irritable Bowel Syndrome with Diarrhea. Dig Dis Sci 57, 1222–1226 (2012). https://doi.org/10.1007/s10620-012-2035-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-012-2035-5