Abstract
Background
The role of peroxisome proliferator-activated receptor delta (PPAR δ) in the development and progression of colorectal cancer (CRC) remains controversial.
Aims
We investigated the impact of PPAR δ expression in tissues on liver metastasis of CRC.
Methods
We analyzed samples of primary CRC and matched normal adjacent tissues from 52 patients for the expression of PPAR δ, cyclooxygenase (COX)-2, vascular endothelial growth factor (VEGF)-A, and CXC chemokine receptor 4 (CXCR4). Correlations of the molecules expressions with clinical characteristics and prognosis of patients were studied.
Results
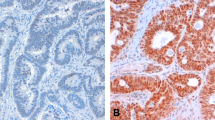
The number of patients positive for PPAR δ, COX-2, CXCR4, and VEGF-A was 25, 33, 18, and 19, respectively. Among the PPAR δ (+)/COX-2 (+), PPAR δ (−)/COX-2 (+), PPAR δ (+)/COX-2 (−), and PPAR δ (−)/COX-2 (−) patient groups, PPAR δ (+)/COX-2 (+) patients had the highest incidence of liver metastasis (p < 0.01). PPAR δ (+)/COX-2 (+) expression was a significant independent prognostic factor (HR = 7.108, 95% CI 1.231–41.029, p = 0.0283) by Cox proportional analysis. PPAR δ (+)/COX-2 (+) patients had the highest positivity for CXCR4 or VEGF-A in tissues (p < 0.01). Among the patients in the CXCR4 (+)/VEGF-A (+), CXCR4 (+)/VEGF-A (−), CXCR4 (−)/VEGF-A (+), and CXCR4 (−)/VEGF-A (−) groups, CXCR4 (+)/VEGF-A (+) patients had the highest incidence of liver metastasis (p < 0.01).
Conclusions
The expression of both PPAR δ and COX-2 in tissues may lead to liver metastasis and consequent poor prognosis in CRC patients.


Similar content being viewed by others
References
Chambers AF, Groom AC, Macdonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572.
Yeatman TJ, Nicolson GL. Molecular basis of tumor progression: mechanisms of organ-specific tumor metastasis. Semin Surg Oncol. 1993;9:256–263.
Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767.
Kliewer SA, Forman BM, Blumberg B, et al. Differential expression and activation of a family of murine peroxisome proliferator-activated receptors. Proc Natl Acad Sci USA. 1994;91:7355–7359.
Harman FS, Nicol CJ, Marin HE, Ward JM, Gonzalez FJ, Peters JM. Peroxisome proliferator-activated receptor-d attenuates colon carcinogenesis. Nat Med. 2004;10:481–483.
Reed KR, Sansom OJ, Hayes AJ, et al. PPAR δ status and Apc-mediated tumourigenesis in the mouse intestine. Oncogene. 2004;23:8992–8996.
Park BH, Vogelstein B, Kinzler KW. Genetic disruption of PPARd decreases the tumorigenicity of human colon cancer cells. Proc Natl Acad Sci USA. 2001;98:2598–2603.
He TC, Chan TA, Vogelstein B, Kinzler KW. PPARd is an APC regulated target of nonsteroidal anti-inflammatory drugs. Cell. 1999;99:335–345.
Gupta RA, Wang D, Katkuri S, Wang H, Dey SK, DuBois RN. Activation of nuclear hormone receptor peroxisome proliferator-activated receptor-d accelerates intestinal adenoma growth. Nat Med. 2004;10:245–247.
Takayama O, Yamamoto H, Damdinsuren B, et al. Expression of PPARd in multistage carcinogenesis of the colorectum: implications of malignant cancer morphology. Br J Cancer. 2006;95:889–895.
Zuo X, Peng Z, Moussalli MJ, et al. Targeted genetic disruption of peroxisome proliferator-activated receptor-delta and colonic tumorigenesis. J Natl Cancer Inst. 2009;101:762–767.
Nakamoto H, Uetake H, Iida S, et al. Correlations between cyclooxygenase-2 expression and angiogenic factors in primary tumors and liver metastases in colorectal cancer. Jpn J Clin Oncol. 2007;37(9):679–685.
Cascinu Stefano, Staccioli MariaPia, Gasparini Giampietro, et al. Expression of vascular endothelial growth factor can predict event-free survival in stage II colon cancer. Clin Cancer Res. 2000;6:2803–2807.
Ottaiano A, Franco R, Talamanca AA, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–2803.
Cutler NS, Graves-Deal R, LaFleur BJ, et al. Stromal production of prostacyclin confers an antiapoptotic effect to colonic epithelial cells. Cancer Res. 2003;63:1748–1751.
Wang D, Mann JR, DuBois RN. WNT and cyclooxygenase-2 crosstalk accelerates adenoma growth. Cell Cycle. 2004;3:1512–1515.
Wang D, Wang H, Shi Q, et al. Prostaglandin E(2) promotes colorectal adenoma growth via transactivation of the nuclear peroxisome proliferator-activated receptor delta. Cancer Cell. 2004;6:285–295.
Gupta RA, Tan J, Krause WF, et al. Prostacyclin-mediated activation of peroxisome proliferator-activated receptor delta in colorectal cancer. Proc Natl Acad Sci USA. 2000;97:13275–13280.
Abdollahi A, Schwager C, Kleeff J, et al. Transcriptional network governing the angiogenic switch in human pancreatic cancer. Proc Natl Acad Sci USA. 2007;104:12890–12895.
Ishizuka M, Sawada T, Okada T, et al. Influence of tumor peroxisome proliferator-activated receptor gamma and delta expression on postoperative mortality of patients undergoing colorectal cancer surgery. J Invest Surg. 2009;22:105–111.
Kato M, Kitayama J, Kazama S, Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:R144–R150.
Uchida D, Begum NM, Almofti A, et al. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp Cell Res. 2003;290:289–302.
Schimanski CC, Schwald S, Simiantonaki N, et al. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin Cancer Res. 2005;11:1743–1750.
Gassmann P, Haier J, Schlüter K, et al. CXCR4 regulates the early extravasation of metastatic tumor cells in vivo. Neoplasia. 2009;11:651–661.
Ohji Y, Yao T, Eguchi T, et al. Evaluation of risk of liver metastasis in colorectal adenocarcinoma based on the combination of risk factors including CD10 expression: multivariate analysis of clinicopathological and immunohistochemical factors. Oncol Rep. 2007;17:525–530.
Zuo X, Wu Y, Morris JS, et al. Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity. Oncogene. 2006;25:1225–1241.
Richard CL, Lowthers EL, Blay J. 15-Deoxy-delta(12, 14)-prostaglandin J(2) down-regulates CXCR4 on carcinoma cells through PPARgamma- and NFkappaB-mediated pathways. Exp Cell Res. 2007;313:3446–3458.
Richard CL, Blay J. Thiazolidinedione drugs down-regulate CXCR4 expression on human colorectal cancer cells in a peroxisome proliferator activated receptor gamma-dependent manner. Int J Oncol. 2007;30:1215–1222.
Wu KK, Liou JY. Cyclooxygenase inhibitors induce colon cancer cell apoptosis Via PPAR delta→gt;14–3-3 epsilon pathway. Methods Mol Biol. 2009;512:295–307.
Acknowledgments
The authors thank Dr. Koji Yoshikawa (Department of pathology, National Hospital Organization Beppu Medical Center, Oita, Japan), Mr. Hitoshi Watanabe (Bioscience Education and Research Center, Akita University, Akita, Japan), and the pathological staff of both of the Beppu Medical Center (Oita, Japan) and Kyodo Byori, Inc. (Hyogo, Japan) for their useful suggestions and technical support about immunohistochemistry. There are no financial disclosures and potential conflicts of interest from any authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yoshinaga, M., Taki, K., Somada, S. et al. The Expression of Both Peroxisome Proliferator-Activated Receptor Delta and Cyclooxygenase-2 in Tissues Is Associated with Poor Prognosis in Colorectal Cancer Patients. Dig Dis Sci 56, 1194–1200 (2011). https://doi.org/10.1007/s10620-010-1389-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1389-9