Abstract
Background
Anticholinergic drugs are commonly prescribed for symptomatic treatment of overactive bladder (OAB). While recent meta-analyses have characterized the prevalence of dry mouth among patients utilizing OAB medications, prevalence of constipation has not been systematically reviewed.
Aims
To provide an effect measure for constipation associated with anticholinergic OAB drugs versus placebo.
Methods
A meta-analysis of trials with darifenacin, fesoterodine, oxybutynin, solifenacin, tolterodine, and trospium was conducted. All randomized, placebo-controlled studies of anticholinergic OAB drugs published in English language and identified in Medline and Cochrane databases were considered for inclusion in this meta-analysis. Those meeting predetermined design characteristics and having sufficient duration (≥2 weeks) were included. Constipation-related data from all included studies were abstracted.
Results
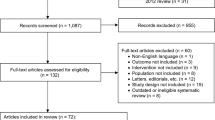
One hundred two English-language, randomized, placebo-controlled trials were originally identified. Thirty-seven studies were ultimately included in the analysis, involving 19,434 total subjects (12,368 treatment + 7,066 placebo patients). The odds ratios for constipation compared with placebo were as follows: overall [odds ratio (OR) 2.18, 95% confidence interval (CI) = 1.82–2.60], tolterodine (OR 1.36, 95% CI = 1.01–1.85), darifenacin (OR 1.93, 95% CI = 1.40–2.66), fesoterodine (OR 2.07, 95% CI = 1.28–3.35), oxybutynin (OR 2.34, 95% CI = 1.31–4.16), trospium (OR 2.93, 95% CI = 2.00–4.28), and solifenacin (OR 3.02, 95% CI = 2.37–3.84).
Conclusions
Our results demonstrate that patients prescribed anticholinergic OAB drugs are significantly more likely to experience constipation. Differences in muscarinic receptor affinities among individual agents may possibly account for the modest variation in constipation rates observed; however, such a determination warrants additional research.


Similar content being viewed by others
References
Stewart WF, Van Rooyen JB, Cundiff GW, et al. Prevalence and burden of overactive bladder in the United States. World J Urol. 2003;20(6):327–336.
Rudd KM, Raehl CL, Bond CA, Abbruscato TJ, Stenhouse AC. Methods for assessing drug-related anticholinergic activity. Pharmacotherapy. 2005;25(11):1592–1601.
Sink KM, Thomas J 3rd, Xu H, Craig B, Kritchevsky S, Sands LP. Dual use of bladder anticholinergics and cholinesterase inhibitors: long-term functional and cognitive outcomes. J Am Geriatr Soc. 2008;56(5):847–853.
Tune LE. Anticholinergic effects of medication in elderly patients. J Clin Psychiatry. 2001;62(Suppl 21):11–14.
D’Souza AO, Smith MJ, Miller LA, Doyle J, Ariely R. Persistence, adherence, and switch rates among extended-release and immediate-release overactive bladder medications in a regional managed care plan. J Manag Care Pharm. 2008;14(3):291–301.
Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California medicaid program. Value Health. 2005;8(4):495–505.
Brubaker L, Fanning K, Goldberg EL, et al. Predictors of discontinuing overactive bladder medications. BJU Int. 2009.
Hay-Smith J, Herbison P, Ellis G, Morris A. Which anticholinergic drug for overactive bladder symptoms in adults. Cochrane Database Syst Rev. 2005;(3):CD005429.
Abrams P, Andersson KE, Buccafusco JJ, et al. Muscarinic receptors: their distribution and function in body systems, and the implications for treating overactive bladder. Br J Pharmacol. 2006;148(5):565–578.
Kay GG, Abou-Donia MB, Messer WS Jr, Murphy DG, Tsao JW, Ouslander JG. Antimuscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc. 2005;53(12):2195–2201.
Kay G, Crook T, Rekeda L, et al. Differential effects of the antimuscarinic agents darifenacin and oxybutynin ER on memory in older subjects. Eur Urol. 2006;50(2):317–326.
Nabi G, Cody JD, Ellis G, Herbison P, Hay-Smith J. Anticholinergic drugs versus placebo for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2006;(4):CD003781.
Choung RS, Locke GR 3rd, Schleck CD, Zinsmeister AR, Talley NJ. Cumulative incidence of chronic constipation: a population-based study 1988–2003. Aliment Pharmacol Ther. 2007;26(11–12):1521–1528.
Shah ND, Chitkara DK, Locke GR, Meek PD, Talley NJ. Ambulatory care for constipation in the United States, 1993–2004. Am J Gastroenterol. 2008;103(7):1746–1753.
Crane SJ, Talley NJ. Chronic gastrointestinal symptoms in the elderly. Clin Geriatr Med. 2007;23(4):721–734.
Spinzi GC. Bowel care in the elderly. Dig Dis. 2007;25(2):160–165.
Singh G, Lingala V, Wang H, et al. Use of health care resources and cost of care for adults with constipation. Clin Gastroenterol Hepatol. 2007;5(9):1053–1058.
Dmochowski RR, Appell RA. Advancements in pharmacologic management of the overactive bladder. Urology. 2000;56(6 Suppl 1):41–49.
Roxburgh C, Cook J, Dublin N. Anticholinergic drugs versus other medications for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2007;(4):CD003190.
Wein AJ. Pharmacologic options for the overactive bladder. Urology. 1998;51(2A Suppl):43–47.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17(1):1–12.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748.
DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188.
Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23(11):1663–1682.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101.
Lipton RB, Kolodner K, Wesnes K. Assessment of cognitive function of the elderly population: effects of darifenacin. J Urol. 2005;173(2):493–498.
Staskin DR, Dmochowski RR, Sand PK, et al. Efficacy and safety of oxybutynin chloride topical gel for overactive bladder: a randomized, double-blind, placebo controlled, multicenter study. J Urol. 2009;181(4):1764–1772.
Van Kerrebroeck P, Kreder K, Jonas U, Zinner N, Wein A. Tolterodine once-daily: superior efficacy and tolerability in the treatment of the overactive bladder. Urology. 2001;57(3):414–421.
The CONSORT Group: CONSORT Statement Website [online]. URL: http://www.consort-statement.org/home; 2010 Accessed 04.03.10.
Ioannidis JP, Evans SJ, Gotzsche PC, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Ann Intern Med. 2004;141(10):781–788.
Alhasso AA, McKinlay J, Patrick K, Stewart L. Anticholinergic drugs versus non-drug active therapies for overactive bladder syndrome in adults. Cochrane Database Syst Rev. 2006;(4):CD003193.
Jonas U, Hofner K, Madersbacher H, Holmdahl TH. Efficacy and safety of two doses of tolterodine versus placebo in patients with detrusor overactivity and symptoms of frequency, urge incontinence, and urgency: urodynamic evaluation. The International Study Group. World J Urol. 1997;15:144–151.
Malone-Lee JG, Walsh JB, Maugourd MF. Tolterodine: a safe and effective treatment for older patients with overactive bladder. J Am Geriatr Soc. 2001;49(6):700–705.
Jacquetin B, Wyndaele J. Tolterodine reduces the number of urge incontinence episodes in patients with an overactive bladder. Eur J Obstet Gynecol Reprod Biol. 2001;98(1):97–102.
Chapple CR, Rechberger T, Al-Shukri S, et al. Randomized, double-blind placebo- and tolterodine-controlled trial of the once-daily antimuscarinic agent solifenacin in patients with symptomatic overactive bladder. BJU Int. 2004;93(3):303–310.
Pfizer, Canada: product monograph—Detrol (tolterodine tablets) [online]. URL: http://www.pfizer.ca/english/our%20products/prescription%20pharmaceuticals/default.asp?s=1&id=8&doc=enmonograph; Accessed 04.03.10.
Appell RA. Clinical efficacy and safety of tolterodine in the treatment of overactive bladder: a pooled analysis. Urology. 1997;50(6A Suppl):90–96; discussion 97–9.
Homma Y, Paick JS, Lee JG, Kawabe K. Clinical efficacy and tolerability of extended-release tolterodine and immediate-release oxybutynin in Japanese and Korean patients with an overactive bladder: a randomized, placebo-controlled trial. BJU Int. 2003;92(7):741–747.
Kaplan SA, Roehrborn CG, Rovner ES, Carlsson M, Bavendam T, Guan Z. Tolterodine and tamsulosin for treatment of men with lower urinary tract symptoms and overactive bladder: a randomized controlled trial. JAMA. 2006;296(19):2319–2328.
Khullar V, Hill S, Laval KU, Schiotz HA, Jonas U, Versi E. Treatment of urge-predominant mixed urinary incontinence with tolterodine extended release: a randomized, placebo-controlled trial. Urology. 2004;64(2):269–274; discussion 274–5.
Rackley R, Weiss JP, Rovner ES, Wang JT, Guan Z. Nighttime dosing with tolterodine reduces overactive bladder-related nocturnal micturitions in patients with overactive bladder and nocturia. Urology. 2006;67(4):731–736; discussion 736.
Novartis Pharmaceutical Corp. Center for Drug Evaluation and Research—application number 21-513; medical review: Enablex (darifenacin) extended release tablets [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-513_Enablex.cfm; Accessed 04.03.10.
Haab F, Stewart L, Dwyer P. Darifenacin, an M3 selective receptor antagonist, is an effective and well-tolerated once-daily treatment for overactive bladder. Eur Urol. 2004;45(4):420–429; discussion 429.
Hill S, Khullar V, Wyndaele JJ, Lheritier K. Dose response with darifenacin, a novel once-daily M3 selective receptor antagonist for the treatment of overactive bladder: results of a fixed dose study. Int Urogynecol J Pelvic Floor Dysfunct. 2006;17(3):239–247.
Steers W, Corcos J, Foote J, Kralidis G. An investigation of dose titration with darifenacin, an M3-selective receptor antagonist. BJU Int. 2005;95(4):580–586.
Chapple C, DuBeau C, Ebinger U, Rekeda L, Viegas A. Darifenacin treatment of patients ≥65 years with overactive bladder: results of a randomized, controlled, 12-week trial. Curr Med Res Opin. 2007;23(10):2347–2358.
Pfizer, Inc. Center for Drug Evaluation and Research—application number 22-030; drug approval package: Toviaz (Fesoterodine Fumarate) tablets [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2008/022030s000TOC.cfm; Accessed 04.03.10.
Chapple C, Van Kerrebroeck P, Tubaro A, et al. Clinical efficacy, safety, and tolerability of once-daily fesoterodine in subjects with overactive bladder. Eur Urol. 2007;52(4):1204–1212.
Nitti VW, Dmochowski R, Sand PK, et al. Efficacy, safety and tolerability of fesoterodine for overactive bladder syndrome. J Urol. 2007;178(6):2488–2494.
Herschorn S, Swift S, Guan Z, et al. Comparison of fesoterodine and tolterodine extended release for the treatment of overactive bladder: a head-to-head placebo-controlled trial. BJU Int. 2010;105(1):58–66.
Szonyi G, Collas DM, Ding YY, Malone-Lee JG. Oxybutynin with bladder retraining for detrusor instability in elderly people: a randomized controlled trial. Age Ageing. 1995;24(4):287–291.
Watson Pharma, Inc. Center for Drug Evaluation and Research—application number 21-351; package label: Oxytrol (oxybutynin transdermal system) [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/label/2003/021351lbl.pdf; Accessed 04.03.10.
Watson Pharma, Inc. Center for Drug Evaluation and Research—application number 21-351; medical review: Oxytrol (oxybutynin transdermal system) [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/21-351_Oxytrol.cfm; Accessed 04.03.10.
Dmochowski RR, Sand PK, Zinner NR, Gittelman MC, Davila GW, Sanders SW. Comparative efficacy and safety of transdermal oxybutynin and oral tolterodine versus placebo in previously treated patients with urge and mixed urinary incontinence. Urology. 2003;62(2):237–242.
Lackner TE, Wyman JF, McCarthy TC, Monigold M, Davey C. Randomized, placebo-controlled trial of the cognitive effect, safety, and tolerability of oral extended-release oxybutynin in cognitively impaired nursing home residents with urge urinary incontinence. J Am Geriatr Soc. 2008;56(5):862–870.
Thuroff JW, Bunke B, Ebner A, et al. Randomized, double-blind, multicenter trial on treatment of frequency, urgency and incontinence related to detrusor hyperactivity: oxybutynin versus propantheline versus placebo. J Urol. 1991;145(4):813–816; discussion 816–7.
Tapp AJ, Cardozo LD, Versi E, Cooper D. The treatment of detrusor instability in post-menopausal women with oxybutynin chloride: a double blind placebo controlled study. Br J Obstet Gynaecol. 1990;97(6):521–526.
Zinner N, Tuttle J, Marks L. Efficacy and tolerability of darifenacin, a muscarinic M3 selective receptor antagonist (M3 SRA), compared with oxybutynin in the treatment of patients with overactive bladder. World J Urol. 2005;23(4):248–252.
Zinner N, Gittelman M, Harris R, Susset J, Kanelos A, Auerbach S. Trospium chloride improves overactive bladder symptoms: a multicenter phase III trial. J Urol. 2004;171(6 Pt 1):2311–2315. (quiz 2435).
Indevus Pharmaceutical, Inc. Center for Drug Evaluation and Research—application number 21-595; drug approval package: Sanctura (Trospium Chloride) extended release capsules [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-595_Sanctura.cfm; Accessed 04.03.10.
Rudy D, Cline K, Harris R, Goldberg K, Dmochowski R. Multicenter phase III trial studying trospium chloride in patients with overactive bladder. Urology. 2006;67(2):275–280.
Staskin D, Sand P, Zinner N, Dmochowski R. Once daily trospium chloride is effective and well tolerated for the treatment of overactive bladder: results from a multicenter phase III trial. J Urol. 2007;178(3 Pt 1):978–983; discussion 983–4.
Indevus Pharmaceutical, Inc. Center for Drug Evaluation and Research—application number 22-103; drug approval package: Sanctura XR (Trospium Chloride) extended release capsules [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2007/022103s000TOC.cfm; Accessed 04.03.10.
Dmochowski RR, Sand PK, Zinner NR, Staskin DR. Trospium 60 mg once daily (QD) for overactive bladder syndrome: results from a placebo-controlled interventional study. Urology. 2008;71(3):449–454.
Yamanouchi Pharma America, Inc. Center for Drug Evaluation and Research—application number 21-518; drug approval package: VesiCare (Solifenacin Succinate) tablets [online]. URL: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2004/21-518_VesiCare.cfm; Accessed 04.03.10.
Cardozo L, Lisec M, Millard R, et al. Randomized, double-blind placebo controlled trial of the once daily antimuscarinic agent solifenacin succinate in patients with overactive bladder. J Urol. 2004;172(5 Pt 1):1919–1924.
Yamaguchi O, Marui E, Kakizaki H, et al. Randomized, double-blind, placebo- and propiverine-controlled trial of the once-daily antimuscarinic agent solifenacin in Japanese patients with overactive bladder. BJU Int. 2007;100(3):579–587.
Cardozo L, Hessdorfer E, Milani R, et al. Solifenacin in the treatment of urgency and other symptoms of overactive bladder: results from a randomized, double-blind, placebo-controlled, rising-dose trial. BJU Int. 2008;102(9):1120–1127.
Karram MM, Toglia MR, Serels SR, Andoh M, Fakhoury A, Forero-Schwanhaeuser S. Treatment with solifenacin increases warning time and improves symptoms of overactive bladder: results from VENUS, a randomized, double-blind, placebo-controlled trial. Urology. 2009;73(1):14–18.
Acknowledgments
This work was performed without financial support. The authors of this paper have no financial interests to declare.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10620_2010_1313_MOESM2_ESM.eps
Fig. S3. Meta-analysis of studies of anticholinergic drugs versus placebo for overactive bladder in the analysis of constipation events (subgroup analyses by treatment duration and trial quality). (EPS 1,043 kb)
Rights and permissions
About this article
Cite this article
Meek, P.D., Evang, S.D., Tadrous, M. et al. Overactive Bladder Drugs and Constipation: A Meta-Analysis of Randomized, Placebo-Controlled Trials. Dig Dis Sci 56, 7–18 (2011). https://doi.org/10.1007/s10620-010-1313-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1313-3