Abstract
Background
Amifostine has been widely tested as a cytoprotective agent against a number of aggressors in different organs. Recently, a gastroprotective effect was observed for this drug in a model of indomethacin-induced gastric injury. Our objective was to investigate the effect of amifostine on ethanol-induced gastric injury and the role played in this mechanism by afferent sensory neurons, non-protein sulfhydryl groups, nitric oxide, ATP-sensitive potassium channels, and cyclooxygenase-2.
Methods
Rats were treated with amifostine (22.5, 45, 90, or 180 mg/kg, PO or SC). After 30 min, the rats received absolute ethanol (5 ml kg−1, PO). One hour later, gastric damage was quantified with a planimeter. Samples from the stomach were also taken for histopathological assessment and for assays of non-protein sulfhydryl groups. The other groups were pretreated with L-NAME (10 mg kg−1, IP), glibenclamide (10 mg kg−1, PO), or celecoxib (10 mg kg−1, PO). After 30 min, the animals were given amifostine (90 mg kg−1, PO or SC), followed 30 min later by gavage with absolute ethanol (5 ml kg−1). Other rats were desensitized with capsaicin (125 mg kg−1, SC) 8 days prior to amifostine treatment.
Results
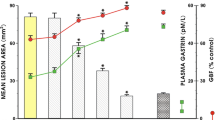
Amifostine administration PO and SC significantly and dose-dependently reduced ethanol-induced macroscopic and microscopic gastric damage by restoring glutathione levels in the stomach mucosa. Amifostine-promoted gastroprotection against ethanol-induced stomach injury was reversed by pretreatment with neurotoxic doses of capsaicin, but not by L-NAME, glibenclamide, or celecoxib.
Conclusions
Amifostine protects against ethanol-induced gastric injury by increasing glutathione levels and stimulating the afferent sensory neurons in the stomach.







Similar content being viewed by others
References
Gottfried EB, Korsten MA, Lieber CS. Alcohol-induced gastric and duodenal lesions in man. Am J Gastroenterol. 1978;70:587–592.
Laine L, Weinstein WM. Histology of alcoholic hemorrhagic “gastritis”: a prospective evaluation. Gastroenterology. 1988;94:1254–1262.
Szabo S, Trier JS, Brown A, Schnoor J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology. 1985;88:228–236.
Evangelista S. Role of sensory neurons in restitution and healing of gastric ulcers. Curr Pharm Des. 2006;12:2977–2984.
Rahgozar M, Pazokitoroudi H, Bakhtiarian A, Djahanguiri B. Diazoxide, a K(ATP) opener, accelerates restitution of ethanol or indomethacin-induced gastric ulceration in rats independent of polyamines. J Gastroenterol Hepatol. 2001;16:290–296.
Konturek SJ, Brzozowski T, Majka J, Pytko-Polonczyk J, Stachura J. Inhibition of nitric oxide synthase delays healing of chronic gastric ulcers. Eur J Pharmacol. 1993;239:215–217.
Medeiros JVR, Bezerra VH, Gomes AS, Barbosa AL, Lima-Junior RC, Soares PM, Brito GA, Ribeiro RA, Cunha FQ, Souza MH. Hydrogen sulphide prevents ethanol-induced gastric damage in mice: role of KATP channels and capsaicin-sensitive primary afferent neurons. J Pharmacol Exp Ther. 2009 (in press).
Kountouras J, Chatzopoulos D, Zavos C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology. 2001;48:743–751.
Konturek SJ, Brzozowski T, Piastucki I, Radecki T, Dupuy D, Szabo S. Gastric mucosal protection by agents altering gastric mucosal sulfhydryls. Role of endogenous prostaglandins. Digestion. 1987;37:67–71.
Capizzi RL. Clinical status and optimal use of amifostine. Oncology. 1999;13:47–59.
Links M, Lewis C. Chemoprotectants: a review of their clinical pharmacology and therapeutic efficacy. Drugs. 1999;57:293–308.
Batista CK, Mota JM, Souza ML, et al. Amifostine and glutathione prevent ifosfamide- and acrolein-induced hemorrhagic cystitis. Cancer Chemother Pharmacol. 2007;59:71–77.
Mota JM, Soares PM, Menezes AA, et al. Amifostine (Wr-2721) prevents indomethacin-induced gastric damage in rats: role of non-protein sulfhydryl groups and leukocyte adherence. Dig Dis Sci. 2007;52:119–125.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem. 1968;25:192–195.
Szabo S, Nagy L, Plebani M. Glutathione, protein sulfhydryls and cysteine proteases in gastric mucosal injury and protection. Clin Chim Acta. 1992;206:95–105.
Maggi CA, Patacchini R, Santicioli P, Giuliani S, Geppetti P, Meli A. Protective action of ruthenium red toward capsaicin desensitization of sensory fibers. Neurosci Lett. 1988;88:201–205.
Ehrlich K, Sicking C, Respondek M, Peskar BM. Interaction of cyclooxygenase isoenzymes, nitric oxide, and afferent neurons in gastric mucosal defense in rats. J Pharmacol Exp Ther. 2004;308:277–283.
Vázquez-Ramírez R, Olguín-Martínez M, Kubli-Garfias C, Hernández-Muñoz R. Reversing gastric mucosal alterations during ethanol-induced chronic gastritis in rats by oral administration of Opuntia ficus-indica mucilage. World J Gastroenterol. 2006;12(27):4318–4324.
Farias-Silva E, Cola M, Calvo TR, et al. Antioxidant activity of indigo and its preventive effect against ethanol-induced DNA damage in rat gastric mucosa. Planta Med. 2007;73(12):1241–1246.
Chow JY, Ma L, Cho CH. Effect of cigarette smoke on ethanol-induced gastric mucosal lesions: the role of nitric oxide and neutrophils. Eur J Pharmacol. 1998;342(2–3):253–260.
Miller TA. Protective effects of prostaglandins against gastric mucosal damage: current knowledge and proposed mechanisms. Am J Physiol. 1983;245:601–623.
Sternini C, Reeve JR, Brecha N. Distribution and characterization of calcitonin-gene-related peptide immunoreactivity in the digestive system of normal and capsaicin-treated rats. Gastroenterology. 1987;93:852–862.
Green T, Dockray GJ. Characterization of the peptidergic afferent innervation of the stomach in the rat, mouse and guinea pig. Neuroscience. 1988;25:181–193.
Abdel-Salam OM, Debreceni A, Mózsik G, Szolcsányi J. Capsaicin-sensitive afferent sensory nerves in modulating gastric mucosal defense against noxious agents. J Physiol Paris. 1999;93(5):443–454.
Abdel Salam OM, Mózsik G, Szolcsányi J. Studies on the effect of intragastric capsaicin on gastric ulcer and on the prostacyclin-induced cytoprotection in rats. Pharmacol Res. 1995;32(4):209–215.
Kwiecień S, Brzozowski T, Konturek PC, et al. The role of reactive oxygen species and capsaicin-sensitive sensory nerves in the pathomechanisms of gastric ulcers induced by stress. J Physiol Pharmacol. 2003;54:423–437.
Souza MH, de Lima OM Jr, Zamuner SR, Fiorucci S, Wallace JL. Gastritis increases resistance to aspirin-induced mucosal injury via COX-2-mediated lipoxin synthesis. Am J Physiol Gastrointest Liver Physiol. 2003;285(1):54–61.
Peskar BM, Ehrlich K, Peskar BA. Role of ATP-sensitive potassium channels in prostaglandin-mediated gastroprotection in the rat. J Pharmacol Exp Ther. 2002;301:969–974.
Acknowledgments
The authors would like to thank Maria Silvandira Freire França and José Ivan Rodrigues for their technical assistance. This study was supported by grants from CNPq (Brazil). Dr. Ribeiro, Dr. Brito, and Dr. Souza are CNPq fellowship holders. This study is part of the requirements for coauthor J. J. Junqueira to obtain a Master of Science degree in Pharmacology from the Medical School at the Federal University of Ceará.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junqueira-Júnior, J., Junqueira, A.F.T.A., Medeiros, J.V.R. et al. Role of Capsaicin-Sensitive Primary Afferent Neurons and Non-protein Sulphydryl Groups on Gastroprotective Effect of Amifostine Against Ethanol-Induced Gastric Damage in Rats. Dig Dis Sci 56, 314–322 (2011). https://doi.org/10.1007/s10620-010-1300-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1300-8