Abstract
Background
Sphingomyelin (SM) is present in dietary products and cell plasma membranes. We previously showed that dietary SM inhibited cholesterol absorption in rats. In the intestinal tract, SM is mainly hydrolyzed by alkaline sphingomyelinase (alk-SMase) to ceramide.
Aims
We investigated the influence of SM and its hydrolytic products ceramide and sphingosine on cholesterol uptake in intestinal Caco-2 cells.
Methods
Micelles containing bile salt, monoolein, and 14C-cholesterol were prepared with or without SM, ceramide, or sphingosine. The micelles were incubated with Caco-2 cells, and uptake of radioactive cholesterol was quantified.
Results
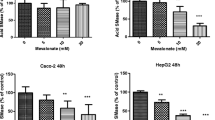
We found that confluent monolayer Caco-2 cells expressed NPC1L1, and the uptake of cholesterol in the cells was inhibited by ezetimibe, a specific inhibitor of NPC1L1. Incorporation of SM in the cholesterol micelles inhibited cholesterol uptake dose-dependently; 38% inhibition occurred at an equal mole ratio of SM and cholesterol. The inhibition was further enhanced to 45% by pretreating the cholesterol/SM micelles with recombinant alk-SMase, which hydrolyzed SM in the micelles by 85%, indicating ceramide has stronger inhibitory effects on cholesterol uptake. To confirm this, we further replaced SM in the micelles with ceramide and sphingosine, and found that at equal mole ratio to cholesterol, ceramide exhibited stronger inhibitory effect (50% vs 38%) on cholesterol uptake than SM, whereas sphingosine only had a weak effect at high concentrations.
Conclusion
Both SM and ceramide inhibit cholesterol uptake, the effect of ceramide being stronger than that of SM. Alk-SMase enhances SM-induced inhibition of cholesterol uptake by generating ceramide in the intestinal lumen.




Similar content being viewed by others
References
Gylling H. Miettinen TA: The effect of cholesterol absorption inhibition on low density lipoprotein cholesterol level. Atherosclerosis. 1995;117:305–308.
Davis HR. Veltri EP: Zetia: Inhibition of niemann-pick c1 like 1 (npc1l1) to reduce intestinal cholesterol absorption and treat hyperlipidemia. J Atheroscler Thromb. 2007;14:99–108.
Dawson PA. Rudel LL: Intestinal cholesterol absorption. Curr Opin Lipidol. 1999;10:315–320.
Blank M, Cress EA, Smith ZL. Snyder F: Meats and fish consumed in the american diet contain substantial amounts of ether-linked phospholipids. J Nutr. 1992;122:1656–1661.
Zeisel SH, Char D. Sheard NF: Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr. 1986;116:50–58.
Nyberg L, Duan RD. Nilsson A: A mutual inhibitory effect on absorption of sphingomyelin and cholesterol. J Nutr Biochem. 2000;11:244–249.
Noh SK. Koo SI: Milk sphingomyelin is more effective than egg sphingomyelin in inhibiting intestinal absorption of cholesterol and fat in rats. J Nutr. 2004;134:2611–2616.
Eckhardt ER, Wang DQ, Donovan JM, Carey MC. Dietary sphingomyelin suppresses intestinal cholesterol absorption by decreasing thermodynamic activity of cholesterol monomers. Gastroenterology. 2002;122:948–956.
Duan RD, Bergman T, Xu N, et al. Nilsson A: Identification of human intestinal alkaline sphingomyelinase as a novel ecto-enzyme related to the nucleotide phosphodiesterase family. J Biol Chem. 2003;278:38528–38536.
Nilsson Å. : The presence of sphingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969;176:339–347.
Duan RD, Hertervig E, Nyberg L, et al. Distribution of alkaline sphingomyelinase activity in human beings and animals. Tissue and species differences. Dig Dis Sci. 1996;41:1801–1806.
Stefan C, Jansen S, Bollen M. Npp-type ectophosphodiesterases: Unity in diversity. Trends Biochem Sci. 2005;30:542–550.
Cheng Y, Nilsson Å, Tömquist E, Duan RD. Purification, characterization and expression of rat intestinal alkaline sphingomyelinase. J Lipid Res. 2002;43:316–324.
Lundgren P, Nilsson Å, Duan RD. Distribution and properties of neutral ceramidase activity in rat intestinal tract. Dig Dis Sci. 2001;46:765–772.
Nilsson A, Duan RD. Absorption and lipoprotein transport of sphingomyelin. J Lipid Res. 2006;47:154–171.
Nyberg L, Duan RD, Axelsson J, Nilsson Å. Identification of an alkaline sphingomyelinase activity in human bile. Biochim Biophys Acta. 1996;1300:42–48.
Andersson D, Kotarsky K, Wu J, Agace W, Duan RD. Expression of alkaline sphingomyelinase in yeast cells and anti-inflammatory effects of the expressed enzyme in a rat colitis model. Dig Dis Sci. 2009;54:1440–1448.
Gibson KM, Hoffmann G, Schwall A, et al. 3-hydroxy-3-methylglutaryl coenzyme a reductase activity in cultured fibroblasts from patients with mevalonate kinase deficiency: differential response to lipid supplied by fetal bovine serum in tissue culture medium. J Lipid Res. 1990;31:515–521.
Field FJ, Shreves T, Fujiwara D, Murthy S, Albright E, Mathur SN. Regulation of gene expression and synthesis and degradation of 3-hydroxy-3-methylglutaryl coenzyme a reductase by micellar cholesterolin caco-2 cells. J Lipid Res. 1991;32:1811–1821.
Chen H, Born E, Mathur SN, Johlin FC, Jr, Field FJ. Sphingomyelin content of intestinal cell membranes regulates cholesterol absorption. Evidence for pancreatic and intestinal cell sphingomyelinase activity. Biochem J. 1992;286((Pt 3)):771–777.
Ikeda I, Matsuoka R, Hamada T, et al. Cholesterol esterase accelerates intestinal cholesterol absorption. Biochim Biophys Acta. 2002;1571:34–44.
Bligh EH, Dyer WJ. A rapid method for total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–918.
Davis HR Jr, Zhu LJ, Hoos LM, et al. Niemann-pick c1 like 1 (npc1l1) is the intestinal phytosterol and cholesterol transporter and a key modulator of whole-body cholesterol homeostasis. J Biol Chem. 2004;279:33586–33592.
Garmy N, Taieb N, Yahi N, Fantini J. Interaction of cholesterol with sphingosine: Physicochemical characterization and impact on intestinal absorption. J Lipid Res. 2005;46:36–45.
Demel RA, Jansen JW, van Dijck PW, van Deenen LL. The preferential interaction of cholesterol with different classes of phospholipids. Biochim Biophys Acta. 1977;465:1–10.
Maulik PR, Shipley GG. N-palmitoyl sphingomyelin bilayers: Structure and interactions with cholesterol and dipalmitoylphosphatidylcholine. Biochemistry. 1996;35:8025–8034.
Ohlsson L, Palmberg C, Duan RD, Olsson M, Bergman T, Nilsson A: Purification and characterization of human intestinal neutral ceramidase. Biochimie 2007.
Nilsson Å. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim Biophys Acta. 1968;164:575–584.
Nyberg L, Nilsson Å, Lundgren P, Duan RD. Localization and capacity of sphingomyelin digestion in the rat intestinal tract. J Nutr Biochem. 1997;8:112–118.
Duan RD. Alkaline sphingomyelinase: An old enzyme with novel implications. Biochim Biophys Acta. 2006;1761:281–291.
Duan RD, Cheng Y, Hansen G, et al. Purification, localization, and expression of human intestinal alkaline sphingomyelinase. J Lipid Res. 2003;44:1241–1250.
Duan RD, Cheng Y, Tauschel HD, Nilsson A. Effects of ursodeoxycholate and other bile salts on levels of rat intestinal alkaline sphingomyelinase: A potential implication in tumorigenesis. Dig Dis Sci. 1998;43:26–32.
Wu J, Liu F, Nilsson A, Duan RD. Pancreatic trypsin cleaves intestinal alkaline sphingomyelinase from mucosa and enhances the sphingomyelinase activity. Am J Physiol Gastrointest Liver Physiol. 2004;287:G967–G973.
Hertervig E, Nilsson A, Nyberg L, Duan RD. Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer. 1997;79:448–453.
Di Marzio L, Di Leo A, Cinque B, et al. Detection of alkaline sphingomyelinase activity in human stool: Proposed role as a new diagnostic and prognostic marker of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:856–862.
Chen H, Born E, Mathur SN, Field FJ. Cholesterol and sphingomyelin syntheses are regulated independently in cultured human intestinal cells, caco-2: Role of membrane cholesterol and sphingomyelin content. J Lipid Res. 1993;34:2159–2167.
Ramstedt B, Slotte JP. Sphingolipids and the formation of sterol-enriched ordered membrane domains. Biochim Biophys Acta. 2006;1758:1945–1956.
Ali MR, Cheng KH, Huang J. Ceramide drives cholesterol out of the ordered lipid bilayer phase into the crystal phase in 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine/cholesterol/ceramide ternary mixtures. Biochemistry. 2006;45:12629–12638.
Acknowledgments
Yajun Cheng is thanked for technical assistance. The work was supported by grants from the Albert Påhlsson Foundation, Swedish Nutrition Foundation, and Research Foundation of Lund University and Lund University Hospital. DF is an exchange researcher from the School of Public Health, Sun Yat-sen University, Guangzhou, China.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Feng, D., Ohlsson, L., Ling, W. et al. Generating Ceramide from Sphingomyelin by Alkaline Sphingomyelinase in the Gut Enhances Sphingomyelin-Induced Inhibition of Cholesterol Uptake in Caco-2 Cells. Dig Dis Sci 55, 3377–3383 (2010). https://doi.org/10.1007/s10620-010-1202-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-010-1202-9