Abstract
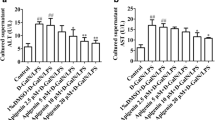
Lipopolysaccharide (LPS) is implicated in the pathology of acute liver injury and can induce lethal liver failure when simultaneously administered with d-galactosamine (d-GalN). At the present time, nonlethal liver failure, the liver injury of clinical implication, is incompletely understood following challenge by low-dose LPS/d-GalN. We report here our investigation of the effects of liver injury following a nonlethal dose LPS/d-GalN and the role of apoptosis in this disorder. Blood biochemistry indexes, including those of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL), had risen by 6 h post-LPS/d-GalN injection, reached a peak at 24 h and sustained high levels at 48 h. An abnormal liver appearance was found at 24 and 48 h post-injection. Histopathological changes of hepatic injuries accompanied by hepatocellular death, inflammatory infiltration and hemorrhage began to appear at 6 h and were markedly aggravated at 24 and 48 h. Cell apoptosis was significantly induced by the nonlethal dose LPS/d-GalN challenge, and the apoptotic indexes (AIs) in 24 h- and 48 h-treated rats were approximately 70%, as estimated by the terminal transferase dUTP nick end labeling (TUNEL) assay. The mRNA levels of the inflammatory cytokine IL-1β rose markedly at 6 h and maintained high levels at 24 and 48 h; however, TNF-α levels were normal in the liver tissues of 6-, 24- and 48-h-treated rats. mRNA expression of the damage gene nitric oxide synthase (NOS) was also induced early by the LPS/d-GalN challenge, reaching a peak at 6 h, then gradually decreasing in a stepwise manner; conversely, high expression levels of the apoptosis-inducing gene p53 mRNA were not found in the early post-injection period (6 h) but emerged in the crest-time of liver apoptosis (24 h) and were maintained at this level until the late stage (48 h). We also observed that in 24 h-treated rats, caspase-3, -8, -9 and -12 were markedly activated by LPS/d-GalN challenge. These results suggest that a challenge with low-dose LPS in conjunction with d-GalN can induce nonlethal but marked liver failure, the main morphological feature of which is hepatic apoptosis, which may be associated with a high expression of inducible (i)NOS (early post-injection period) and p53 genes (in the mid and late stages) and at least three apoptosis pathways participate in the pathogenesis.








Similar content being viewed by others
References
Morrison DC, Danner RL, Dinarello CA et al (1994) Bacterial endotoxins and pathogenesis of Gram-negative infections: current status and future direction. J Endotoxin Res 1:71–83
Thirunavukkarasu C, Uemura T, Wang LF et al (2005) Normal rat hepatic stellate cells respond to endotoxin in LBP-independent manner to produce inhibitor(s) of DNA synthesis in hepatocytes. J Cell Physiol 204:654–665
Wang P, Chaudry IH (1996) Mechanism of hepatocellular dysfunction during hyperdynamic sepsis. Am J Physiol Regulatory Integrative Comp Physiol 270: R927–R938
Bone RC, Balk RA, Cerra FB et al (1992) Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American college of chest physicians/society of critical care medicine. Chest 101:1644–1655
Galanos C, Freudenburg MA, Reutter W (1979) Galactosamine-induced sensitization to the lethal effect of endotoxin. Proc Natl Acad Sci USA 76:5939–5943
Liu D, Li C, Chen Y et al (2004) Nuclear import of proinflammatory transcription factors is required for massive liver apoptosis induced by bacterial lipopolysaccharide. J Biol Chem 279(46):48343–48442
Lehmann V, Freudenberg MA, Galanos C (1987) Lethal toxicity of lipopolysaccharide and tumor necrosis factor in normal and D-GalN-treated mice. J Exp Med 165:657–663
Morikawa A, Kato Y, Sugiyama T et al (1999) Role of nitric oxide in lipopolysaccharide-induced hepatic injury in d-galactosamine-sensitized mice as an experimental endotoxic shock model. Infect Immun 67:1018–1024
Dien MV, Takahashi K, Mu MM et al (2001) Protective effect of Wogonin on endotoxin-induced lethal shock in d-galactosamine-sensitized mice. Microbiol Immunol 45:751–756
Thorlacius K, Slotta JE, Laschke MW et al (2006) Protective effect of fasudil, a Rho-kinase inhibitor, on chemokine expression, leukocyte recruitment, and hepatocellular apoptosis in septic liver injury. J Leukoc Biol 79:923–931
Motobu M, Amer S, Koyama Y et al (2006) Protective effect of sugar cane extract on endotoxic shock in mice. Phytother Res 20(5):359–363
Xiong Q, Hase K, Tezuka Y et al (1999) Acteoside inhibits apoptosis in d-galactosamine and lipopolysaccharide-induced liver injury. Life Sci 65:421–430
Freudenberg MA, Keppler D, Galanos C (1986) Requirement for lipopolysaccharide-responsive macrophages in galactosamine-induced sensitization to endotoxin. Infect Immun 51:891–895
Yin XM, Ding WX (2003) Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr Mol Med 3:491–508
Oreopoulos GD, Wu H, Szaszi K et al (2004) Hypertonic preconditioning prevents hepatocellular injury following ischemia/reperfusion in mice: a role for interleukin 10. Hepatology 40:211–220
McCarter SD, Akyea TG, Lu X et al (2004) Endogenous heme oxygenase induction is a critical mechanism attenuating apoptosis and restoring microvascular perfusion following limb ischemia/reperfusion. Surgery 136:67–75
Ando K, Hiroishi K, Kaneko T et al (1997) Perfoli, Fas/Fas ligand and TNF-α pathways as specific and bystander killing mechanism of hepatic C virus-specific human CTL. J Immunol 158:5283–5291
Yin M, Wheeler MD, Kono H et al (1999) Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology 117:942–952
Gantner F, Leist M, Lohse AW et al (1995) Concanavalin A-induced T-cell-mediated hepatic injury in mice: the role of tumor necrosis factor. Hepatology 21:190–198
Kobayashi Y, Mori M, Naruto T et al (2004) Dynamic movement of cytochrome C from mitochondria into cytosol and peripheral circulation in massive hepatic cell injury. Pediatr Int 46:685–692
Sass G, Koerber K, Bang R et al (2001) Inducible nitric oxide synthase is critical for immune-mediated liver injury in mice. J Clin Invest 107:439–447
Haupt S, Berger M, Goldberg Z et al (2003) Apoptosis – the p53 network. J Cell Sci 116:4077–4085
Thomson RK, Arthur MJ (1999) Mechanisms of liver cell damage and repair. Eur J Gastroenterol Hepatol 11:949–955
Riordan SM, Williams R (2003) Mechanisms of hepatocyte injury, multiorgan failure, and prognostic criteria in acute liver failure. Semin Liver Dis 23:203–215
Jaeschke H, Gujral JS, Bajt ML (2004) Apoptosis and necrosis in liver disease. Liver Int 24:85–99
Kasahara I, Saitoh K, Nakamura K (2000) Apoptosis in acute hepatic failure: histopathological study of human liver tissue using the tunnel method and immunohistochemistry. J Med Dent Sci 47:167–175
Doggrell SA (2004) Suramin: potential in acute liver failure. Expert Opin Investig Drugs 13:1361–1363
Togo S, Kubota T, Matsuo K et al (2004) Mechanisms of liver failure after hepatectomy. Nippon Geka Gakkai Zasshi 105:658–663
Eichhorst ST (2005) Modulation of apoptosis as a target for liver disease. Expert Opin Ther Targets 9:83–99
Kobayashi M, Tsuijitani S, Kurisu Y et al (2002) Bcl-2 and Bax expression for hepatocellular apoptosis in a murine endotoxin shock model. Hepatogastroenterology 49:1602–1606
Leist M, Gantner F, Brohlinger I et al (1995) Tumor necrosis factor-induced hepatocyte apoptosis precedes liver failure in experimental murine shock models. Am J Pathol 146:1220–1234
Morikawa A, Sugiyama T, Kato Y et al (1996) Apoptosis cell death in the response of d-galactosamine-sensitized mice to lipopolysaccharide as an experimental endotoxic shock model. Infect Immun 64:737–738
Kim Y-M, Bergonia H, Lancaster JR (1995) Nitrogen oxide-induced autoprotection in isolated rat hepatocytes. FEBS Lett 374:228–232
Kroncke K-D, Fehsel K, Kolb-Bachofen V (1997) Nitric oxide: cytotoxicity versus cytoprotection- how, why, when and where? Nitric Oxide Biol Chem 1:107–120
Bonfoco E, Krainc D, Ankarcrona M et al (1995) Apoptosis and necrosis: two distinct events induced, respectively, by mild and intense insults with NMDA or nitric oxide/superoxide in cortical cell cultures. Proc Natl Acad Sci USA 92:7162–7166
Lin K, Xue JY, Nomen M et al (1995) Peroxynitrite-induced apoptosis in HL-60 cells. J Biol Chem 270:16487–16490
Ferri KF, Kroemer G (2001) Organelle-specific initiation of cell death pathways. Nat Cell Biol 3:E225–E263
Scorrano L, Korsmeyer SJ (2003) Mechanisms of cytochrome C release by proapoptotic Bcl-2 family members. Biochem Biophys Res Commun 304:437–444
Acknowledgements
We thank Jiang-jin Xu, Yu-chen Dai, Xiao-hua Qu, Jian-hong Jiang and Yai-qing Zou for valuable technical support. This study was supported by National Natural Scientific Grant of China (No.30660066, No.30360037)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, LM., Zhang, JX., Luo, J. et al. A Role of Cell Apoptosis in Lipopolysaccharide (LPS)-induced Nonlethal Liver Injury in d-galactosamine (d-GalN)-sensitized Rats. Dig Dis Sci 53, 1316–1324 (2008). https://doi.org/10.1007/s10620-007-9994-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-007-9994-y