Abstract
We studied clinical and laboratory effects of 3 months of lamivudine with adefovir combination and adefovir dipivoxil (AD) alone in the treatment of patients with lamivudine-resistant hepatitis B virus (HBV) infection. Eligible patients were hepatitis B surface antigen-positive men and women with compensated liver disease who were given lamivudine at least more than 6 months and had HBV polymerase gene mutation. Patients were assigned to receive adefovir 10 mg/day (Group 1) or adefovir 10 mg once daily and lamivudine 100 mg once daily combination during first 3 months, and then stopped lamivudine and continued adefovir (Group 2).
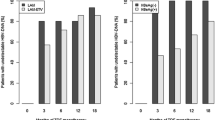
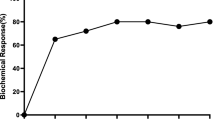
Median age was 48 years (34 males and 20 females, and 35 were HBeAg-negative). Baseline median ALT, AST, and HBV DNA levels were 66 IU/l, 49 IU/l, and 6.7 log10 copy/ml, respectively. Median adefovir therapy time and ALT normalization time were 9 and 3.5 months, respectively. There was no significant difference between groups according to the baseline HBV DNA, ALT, HBe Ag status, age, gender, and lamivudine resistance time. Virological and biochemical responses were similar in both groups during therapy. Two patients (8%) had ALT flare more than five times upper limit of normal without any clinical decompensation in Group 1. Mild ALT elevation according to baseline levels were found in 8 (27.6%) and 4 (17.4%) patients, respectively, in Group 2 and Group 1, and no statistically significance between two groups.
In conclusion, this study showed that it is not necessary to continue lamivudine therapy while switching to AD therapy. Adefovir alone is effective in the treatment of patients with lamivudine resistant HBV infection and compensated liver disease, without significant clinical and laboratory flares. However, it is not easy to say that switching to AD with cessation of lamivudine is safe, because the study population is not enough for precise conclusion and resistance may be a considerable problem against AD in patients using long-term treatment.


Similar content being viewed by others
References
Tassopoulos NC, Anagnostopoulos GD (2000) Long-term lamivudine treatment of patients with precore mutant profile [HBe-Ag(-)/antiHBe (_)] chronic hepatitis B [abstract]. Antivir Ther 5:34
Lau DT, Khokhar MF, Doo E, Ghany MG, Herion D, Park Y, Kleiner DE, Schmid P, Condreay LD, Gauthier J, Kuhns MC, Liang TJ, Hoofnagle JH (2000) Long-term therapy of chronic hepatitis B with lamivudine. Hepatology 32(4 Pt 1):828–834
Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW (2004) Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol 19:1276–1282
Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON, Dixon JS, Gray DF, Sabbat J; Cirrhosis Asian Lamivudine Multicentre Study Group (2004) Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med 351:1521–1531
Leung NW, Lai CL, Chang TT, Guan R, Lee CM, Ng KY, Lim SG, Wu PC, Dent JC, Edmundson S, Condreay LD, Chien RN; on behalf of the Asia Hepatitis Lamivudine Study Group (2001) Extended lamivudine treatment in patients with chronic hepatitis B enhances hepatitis B e antigen seroconversion rates: results after 3 years of therapy. Hepatology 33:1527–1532
Marcellin P, Chang T-T, Lim GS, Tong MJ, Sievert W, Shiffman ML, Jeffers L, Goodman Z, Wulfsohn MS, Xiong S, Fry J, Brosgart CL (2003) Adefovir dipivoxil for the treatment of hepatitis B e antigen positive chronic hepatitis B. N Engl J Med 348:808–816
Hadziyannis SJ, Tassopoulos NC, Heathcote EJ, Chang T-T, Kitis G, Rizzetto M, Marcellin P, Lim SG, Goodman Z, Wulfsohn MS, Xiong S, Fry J, Brosgart CL (2003) Adefovir dipivoxil for the treatment of hepatitis B e antigen-negative chronic hepatitis B. N Engl J Med 348:800–807
Xiong X, Flores C, Yang H, Toole JJ, Gibs CS (1998) Mutations in hepatitis B DNA polymerase associated with resistance to lamivudine do not confer resistance to adefovir in vitro. Hepatology 28:1669–1673
Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A (2000) Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology 32:129–134
Benhamou Y, Bochet M, Thibault V, Calvez V, Fievet MH, Vig P, Gibbs CS, Brosgart C, Fry J, Namini H, Katlama C, Poynard T (2001) Safety and efficacy of adefovir dipivoxil in patients co-infected with HIV-1 and lamivudine-resistant hepatitis B virus: an open label pilot study. Lancet 358:718–723
Perillo R, Hann HW, Mutimer D, Willems B, Leung N, Lee WM, et al. (2004) Adefovir dipivoxil added to ongoing lamivudine in chronic hepatitis B with YMDD mutant hepatitis B virus. Gastroenterology 126: 81–90
Peters MG, Hann HW, Martin P, Heathcote EJ, Buggisch P, Rubin R, et al. (2004) Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology 126: 91–101
Schiff EU, Lai CL, Hadziyannis S, et al. (2003) Adefovir dipivoxil therapy for lamivudine resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology 38:1419
Hosaka T, Suzuki F, Suzuki Y, Saitoh S, Kobayashi M, Someya T, et al. (2004) Adefovir dipivoxil for treatment of breakthrough hepatitis caused by lamivudine-resistant mutants of hepatitis B virus. Intervirology 47:362–369
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akyildiz, M., Gunsar, F., Ersoz, G. et al. Adefovir Dipivoxil Alone or in Combination with Lamivudine for Three Months in Patients with Lamivudine Resistant Compensated Chronic Hepatitis B. Dig Dis Sci 52, 3444–3447 (2007). https://doi.org/10.1007/s10620-006-9718-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-006-9718-8