Abstract
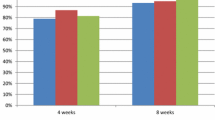
The objective of this trial was to compare the efficacy of esomeprazole, 20 mg, with that of omeprazole, 20 mg, in patients with erosive esophagitis (EE). In this multicenter, double-blind, parallel-group trial, 1176 patients with EE confirmed by endoscopy (Helicobacter pylori-negative by serology) were randomized to once-daily treatment with 20 mg esomeprazole or 20 mg omeprazole for 8 weeks. The primary outcome was the proportion of patients with healed EE through week 8. Secondary outcomes included diary and investigator assessments of heartburn symptoms. Cumulative life-table healing rates at week 8 were similarly high for 20 mg esomeprazole (90.6%; 95% confidence interval, 88.1%–93%) and 20 mg omeprazole (88.3%; 95% confidence interval, 85.5%–91.0%). The two treatments were comparable for other secondary measures and had similar tolerability profiles.
Similar content being viewed by others
References
Spechler SJ (1992) Epidemiology and natural history of gastro-oesophageal reflux disease. Digestion 51(Suppl 1):24–29
Rai A, Orlando R (1998) Gastroesophageal reflux disease. Curr Opin Gastroenterol 14:326–333
Thomson AB, Chiba N, Armstrong D, Tougas G, Hunt RH (1998) The Second Canadian Gastroesophageal Reflux Disease Consensus: Moving forward to new concepts. Can J Gastroenterol 12(8):551–556
Orlando RC (1997) The pathogenesis of gastroesophageal reflux disease: The relationship between epithelial defense, dysmotility, and acid exposure. Am J Gastroenterol 92(Suppl 4):3s–7s
Holloway RH, Dent J, Narielvala F, Mackinnon AM (1996) Relation between oesophageal acid exposure and healing of oesophagitis with omeprazole in patients with severe reflux oesophagitis [see comments]. Gut 38(5):649–654
Freston JW, Malagelada JR, Petersen H, McCloy RF (1995) Critical issues in the management of gastroesophageal reflux disease. Eur J Gastroenterol Hepatol 7(6):577–586
Chiba N, De Gara CJ, et al. (1997) Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology 112(6):1798–1810
Andersson T, Hassan-Alin M, Hasselgren G, Róhss K, Weidolf W (2001) Pharmacokinetic studies with esomeprazole, the (S)-isomer of omeprazole. Clin Pharmacokinet 40(6):411–426
Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche JP, Lundell L, Margulies M, Richter JE, Spechler SJ, Tytgat GN, Wallin L (1996) The endoscopic assessment of esophagitis: A progress report on observer agreement. Gastroenterology 111(1):85–92
Lundell LR, Dent J, Bennett JR, Blum AL, Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler SJ, Tytgat GN, Wallin L (1999) Endoscopic assessment of oesophagitis: Clinical and functional correlates and further validation of the Los Angeles classification. Gut 45(2):172–180
Dent J, Brun J, Fendrick, Fennerty MB, Janssens J, Kahrilas PJ, Lauritsen K, Reynolds JC, Shaw M, Talley NJ, on behalf of the Genval Workshop Group (1999) An evidence-based appraisal of reflux disease management—The Genval Workshop report. Gut 44(Suppl 2):S1–S16
Sontag SJ, Hirschowitz BI, Holt S, Robinson MG, Behar J, Berenson MM, McCullough A, Ippoliti AF, Richter JE, Ahtaridis G, McCallum R, Pambianco DJ, Vlahcevic RZ, Johnson DA, Collen MJ, Lyon DT, Humphries TJ, Cagliola A, Berman RS (1992) Two doses of omeprazole versus placebo in symptomatic erosive esophagitis: The U.S. Multicenter Study. Gastroenterology 102(1):109–118
Corinaldesi R, Valentini M, Belaiche J, Colin R, Geldof H, Maier C (1995) Pantoprazole and omeprazole in the treatment of reflux oesophagitis: A European multicentre study. Aliment Pharmacol Ther 9(6):667–671
Dekkers CP, Beker JA, Thjodleifsson B, Gabryelewicz A, Bell NE, Humphries TJ, the European Rabeprazole Study Group (1999) Double-blind, placebo-controlled comparison of rabeprazole 20 mg vs. 20 mg omeprazole in the treatment of erosive or ulcerative gastro-oesophageal reflux disease. Aliment Pharmacol Ther 13(1):49–57
Sharma VK, Leontiadis GI, Howden CW (2001) Meta-analysis of randomized controlled trials comparing standard clinical doses of omeprazole and lansoprazole in erosive oesophagitis. Aliment Pharmacol Ther 15(2):227–231
Kahrilas PJ, Falk GW, Johnson DA, Schmitt C, Collins DW, Whipple J, D’Amico D, Hamelin B, Joelsson B, for the Esomeprazole Study Investigators (2000) Esomeprazole improves healing and symptom resolution as compared with omeprazole in reflux oesophagitis patients: A randomized controlled trial. Aliment Pharmacol Ther 14(10):1249–1258
Lind T, Rydberg L, Kylebäck A, Jonsson A, Andersson T, Hasselgren G, Holmberg J, Röhss K (2000) Esomeprazole provides improved acid control versus omeprazole in patients with symptoms of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 14(7):861–867
Bell NJV, Burget D, Howden CW, Wilkinson J, Hunt RH (1992) Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion 51(Suppl 1):59–67
Acknowledgments
The authors thank Robert Genta, M.D. (Houston, TX), and Roberto Fiocia, M.D., and Guido Rindo, M.D. (Pavia, Italy), for evaluation of slides; Caroline Spencer and Mary Wiggin for editorial assistance (supported by AstraZeneca); the study coordinators and patients for their participation; and the following trial investigators—David Aarons, Lodi, CA; H. Steven Aharonian, Mission Hills, CA; Ajit Arora, Fresno, CA; Richard D. Baerg, Tacoma, WA; Jay Beckwith, Fort Worth, TX; William Berry, Longmont, CO; William Bray, Charlotte, NC; Steven W. Carlson, San Luis Obispo, CA; Richard Chasen, Laurel, MD; An Yu Chen, Monroe, WI; Yang Chen, Loma Linda, CA; Gene Chiao, Indianapolis, IN; Nicolas Cirillo, Wilkesboro, NC; Charles Colip, Portland, OR; Florian Cortese, Butte, MT; Michael G. DeLissio, Cary, NC; Vincent DeLuca, Derby, CT; James Dimitroff, St. Louis, MO; Ben Dolin, Peoria, IL; Mark Eisner, Zephyrills, FL; Michael Epstein, Annapolis, MD; Gary Falk, Cleveland, OH; Steven Fein, Pasadena, TX; Robert Finlaw, Puebleo, CO; Ronald Fogel, Detroit, MI; Harvey Giller, Clive, IA; Howard Gogel, Albuquerque, NM; Michael Goodman, Chattanooga, TN; Eugene Hirsh, Atlanta, GA; Lynne Hopkins, Winter Park, FL; Keith Hussey, Naples, FL; Rokay Kamyar, La Mesa, CA; Seymour Katz, Great Neck, NY; Lloyd King, Decatur, GA; Ross Kommor, Marietta, GA; Robert Kornfield, Rochester, NY; Thomas Kovacs, Los Angeles, CA; Steven Krumholz, West Palm Beach, FL; Daniel Kruss, Oak Park, IL; Mark Lamet, Hollywood, FL; Frank Lanza, Houston, TX; Loth E. Lieberstein, Reno, NV; James Linne, Cincinnati, OH; Stefan Marcuard, Greenville, NC; Paul Maton, Oklahoma City, OK; Richard McCallum, Kansas City, KS; Hooshang Meshkinpour, Orange, CA; Sam E. Moussa, Tucson, AZ; Rao Movva, Moline, IL; Lanning Newell, Raleigh, NC; Trent Nichols, Jr., Hanover, PA; James S. Novick, Baltimore, MD; Calvin Olson, Modesto, CA; Terryl Jean Ortego, Springdale, AR; Marcos Pedrosa, Boston, MA; J. Mark Provenza, Shreveport, LA; Charles Randall, San Antonio, TX; Roderick Rapier, San Diego, CA; Jean-Pierre Raufman, Little Rock, AR; Adisesha Reddy, Tuscaloosa, AL; Dennis S. Riff, Anaheim, CA; Mark Ringold, Oklahoma City, OK; Gary Rosman, Egg Harbor Township, NJ; Herbert A. Rubin, Beverly Hills, CA; Vinod Rustgi, Fairfax, VA; Bruce Sahba, San Diego, CA; James Scheiman, Ann Arbor, MI; Jerrold L. Schwartz, Arlington Heights, IL; Ronald Schwarz, Raleigh NC; Ann Silverman, Royal Oak, MI; David R. Silvers, Metairier, LA; Sudeep Singh, Fresno, CA; Jack Soterakis, Manhasset, NY; Richard Stellar, Merrick, NY; Abdul Thannoun, Amarillo, TX; James Wagonfield, Tacoma, WA; Steven Wilkofsky, Dallas, TX; John Winder, Sylvania, OH; Robert Wohlman, Bellevue, WA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lightdale, C.J., Schmitt, C., Hwang, C. et al. A Multicenter, Randomized, Double-Blind, 8-Week Comparative Trial of Low-Dose Esomeprazole (20 mg) and Standard-Dose Omeprazole (20 mg) in Patients with Erosive Esophagitis. Dig Dis Sci 51, 852–857 (2006). https://doi.org/10.1007/s10620-005-9071-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-9071-3