Abstract
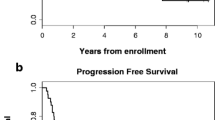
Chemotherapy options for esophagogastric adenocarcinoma remain limited. Irinotecan has demonstrated broad activity in a variety of epithelial malignancies. Forty-six patients with previously untreated, measurable, unresectable, or metastatic esophagogastric adenocarcinoma were enrolled. Patients received irinotecan (125 mg/m2intravenously over 90 min weekly) for 4 consecutive weeks followed by a 2-week rest. Forty-three patients received at least one treatment and were evaluable for response and toxicity. One complete and five partial responses were observed, for an overall response rate of 14% (95% CI, 4–24%). Median survival for all 43 patients was 6.4 months (95% CI, 4.6–8.2 months). Grade 3 to 4 toxicity included 10 patients (23%) with neutropenia, 13 patients (30%) with late diarrhea, 6 patients (14%) with vomiting, and 6 patients (14%) with fatigue. We conclude that although single-agent irinotecan is an active agent for esophagogastric adenocarcinoma, the schedule utilized in this trial is associated with moderate toxicity. When used as a single-agent, a triweekly schedule may be preferable for this patient population.
Similar content being viewed by others
References
Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ: Cancer statistics, 2005 CA Cancer J Clin 55:10–30, 2005
Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ: Cancer statistics, 2004. CA Cancer J Clin 54:8–29, 2004
Fuchs CS, Mayer RJ: Gastric carcinoma. N Engl J Med 333:32–41, 1995
Enzinger PC, Mayer RJ: Esophageal cancer. N Engl J Med 349:2239–2250, 2003
Ajani JA, Ilson DH, Daugherty K, Kelsen DP: Paclitaxel in the treatment of carcinoma of the esophagus. Semin Oncol 22:35–40, 1995
Enzinger PC, Ilson DH, Kelsen DP: Chemotherapy in esophageal cancer. Semin Oncol 26:12–20, 1999
Ilson DH, Saltz L, Enzinger P, Huang Y, Kornblith A, Gollub M, O'Reilly E, Schwartz G, DeGroff J, Gonzalez G, Kelsen DP: Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol 17:3270–3275, 1999
Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, Charnsangavej C: CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer 94:641–646, 2002
Sawada S, Okajima S, Aiyama R, Nokata K, Furuta T, Yokokura T, Sugino E, Yamaguchi K, Miyasaka T: Synthesis and antitumor activity of 20(S)-camptothecin derivatives: carbamate-linked, water-soluble derivatives of 7-ethyl-10-hydroxycamptothecin. Chem Pharm Bull (Tokyo) 39:1446–1450, 1991
Sawada S, Nokata K, Furuta T, Yokokura T, Miyasaka T: Chemical modification of an antitumor alkaloid camptothecin: synthesis and antitumor activity of 7-C-substituted camptothecins. Chem Pharm Bull (Tokyo) 39:2574–2580, 1991
Sawada S, Matsuoka S, Nokata K, Nagata H, Furuta T, Yokokura T, Miyasaka T: Synthesis and antitumor activity of 20(S)-camptothecin derivatives: A-ring modified and 7,10-disubstituted camptothecins. Chem Pharm Bull (Tokyo) 39:3183–3188, 1991
Yaegashi T, Nokata K, Sawada S, Furuta T, Yokokura T, Miyasaka T: Chemical modification of an antitumor alkaloid, 20(S)-camptothecin: glycosides, phosphates and sulfates of 7-ethyl-10-hydroxycamptothecin. Chem Pharm Bull (Tokyo) 40:131–135, 1992
Hsiang YH, Hertzberg R, Hecht S, Liu LF: Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem 260:14873–14878, 1985
Hsiang YH, Liu LF: Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res 48:1722–1726, 1988
Matsuoka H, Furusawa M, Tomoda H, Seo Y, Kono A, Takeda S, Sato K: Cytotoxicity of CPT-11 and SN-38 for gastrointestinal and recurrent carcinomas cultured on contact-sensitive plates. Anticancer Res 14:405–409, 1994
Matsuoka H, Yano K, Seo Y, Saito T, Tomoda H, Takiguchi S, Kono A: Cytotoxicity of CPT-11 for gastrointestinal cancer cells cultured on fixed-contact-sensitive plates. Anticancer Drugs 6:413–418, 1995
Futatsuki K, Wakui A, Nakao I, Sakata Y, Kambe M, Shimada Y, Yoshino M, Taguchi T, Ogawa N: [Late phase II study of irinotecan hydrochloride (CPT-11) in advanced gastric cancer CPT-11. Gastrointestinal Cancer Study Group]. Gan To Kagaku Ryoho 21:1033–1038, 1994
Pitot HC, Wender DB, O'Connell MJ, Schroeder G, Goldberg RM, Rubin J, Mailliard JA, Knost JA, Ghosh C, Kirschling RJ, Levitt R, Windschitl HE: Phase II trial of irinotecan in patients with metastatic colorectal carcinoma. J Clin Oncol 15:2910–2919, 1997
Rothenberg ML, Eckardt JR, Kuhn JG, Burris HA 3rd, Nelson J, Hilsenbeck SG, Rodriguez GI, Thurman AM, Smith LS, Eckhardt SG, Weiss GR, Elfring GL, Rinaldi DA, Schaaf LJ, Von Hoff DD: Phase II trial of irinotecan in patients with progressive or rapidly recurrent colorectal cancer. J Clin Oncol 14:1128–1135, 1996
Rothenberg ML, Cox JV, DeVore RF, Hainsworth JD, Pazdur R, Rivkin SE, Macdonald JS, Geyer CE Jr, Sandbach J, Wolf DL, Mohrland JS, Elfring GL, Miller LL, Von Hoff DD: A multicenter, phase II trial of weekly irinotecan (CPT-11) in patients with previously treated colorectal carcinoma. Cancer 85:786–795, 1999
Fleming TR: One-sample multiple testing procedure for phase II clinical trials. Biometrics 38:143–151, 1982
Kaplan E, Meier P: Nonparametric estimation for incomplete observation. J Am Stat Assoc 53:457–481, 1958
Kohne CH, Catane R, Klein B, Ducreux M, Thuss-Patience P, Niederle N, Gips M, Preusser P, Knuth A, Clemens M, Bugat R, Figer I, Shani A, Fages B, Di Betta D, Jacques C, Wilke HJ: Irinotecan is active in chemonaive patients with metastatic gastric cancer: a phase II multicentric trial. Br J Cancer 89:997–1001, 2003
Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR: Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21:807–814, 2003
Egner J, Goldberg RM, Sargent D, Mahoney M, Richardson R, Miller L: CPT-11 at 320mg/m2 caused excessive toxicity in patients (pts) with advanced adenocarcinoma (ACA) of the stomach (S) or gastroesophageal junction (GJ): A North Central Cancer Treatment Group Trial. Proc Am Soc Clin Oncol 18:282a, 1999
Ajani JA, Baker J, Pisters PW, Ho L, Mansfield PF, Feig BW, Charnsangavej C: Irinotecan/cisplatin in advanced, treated gastric or gastroesophageal junction carcinoma. Oncology (Huntingt) 16:16–18, 2002
Pozzo C, Bugat R, Peschel C, Gorbunova V, Valvere V, Zaluski J, Biakhov M, Barone C: Irinotecan in combination with CDDP or 5-FU and folinic acid is active in patients with advanced gastric or gastro-oesophageal junction adenocarcinoma: Final results of a randomised phase II study. Proc Am Soc Clin Oncol 20:A531, 2001
Raoul J, Bouche O, Giovannini M, Etienne P-L, Bedenne L, Lledo G, Arsene D, Paitel J: Randomized phase II trial of LV5FU2, LV5FU-cisplatinum or LV5FU2-irinotecan in patients (pts) with metastatic gastric or cardial adenocarcinoma (MGA): Final results of study FFCD 9803. Eur J Cancer Suppl 1:S18, 2003
Hawkins R, Cunningham D, Soerbye H, Adenis A, Canon JL, Lopez-Vivanco G, Jacques C, Stenger P: Randomized phase II trial of docetaxel plus irinotecan versus docetaxel plus 5-fluorouracil (5FU) in patients with untreated advanced gastric adenocarcinoma (AGAC). Proc Am Soc Clin Oncol 22:A1032, 2003
Jatoi A, Tirona MT, Cha SS, Alberts SR, Rowland KM, Morton RF, Nair S, Kardinal CG, Stella PJ, Mailliard JA, Sargen D, Goldberg RM: A phase II trial of docetaxel and CPT-11 in patients with metastatic adenocarcinoma of the esophagus, gastroesophageal junction, and gastric cardia. Int J Gastrointest Cancer 32:115–123, 2002
Lordick F, von Schilling C, Bernhard H, Hennig M, Bredenkamp R, Peschel C: Phase II trial of irinotecan plus docetaxel in cisplatin-pretreated relapsed or refractory oesophageal cancer. Br J Cancer 89:630–633, 2003
Souglakos J, Syrigos K, Potamianou A, Polyzos A, Andoulakis N, Vardakis N, Macheras A, Kouroussis: Combination with irinotecan (CPT-11) plus oxaliplatin (L-OHP) as first line treatment in metastatic gastric cancer: A multicenter phase II trial. Proc Am Soc Clin Oncol 22:A1288, 2003
Bamias A, Papamichael D, Syrigos K, Pavlidis N: Phase II study of irinotecan and mitomycin C in 5-fluorouracil-pretreated patients with advanced colorectal and gastric cancer. J Chemother 15:275–281, 2003
Livingston R, Edwards J, Gold P: Phase II trial of irinotecan (CPT-11) and mitomycin c (MMC) in the treatment of metastatic esophageal cancer. Proc Am Soc Clin Oncol 22:A1171, 2003
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was supported by Pfizer Oncology, Inc.
Rights and permissions
About this article
Cite this article
Enzinger, P.C., Kulke, M.H., Clark, J.W. et al. A Phase II Trial of Irinotecan in Patients with Previously Untreated Advanced Esophageal and Gastric Adenocarcinoma. Dig Dis Sci 50, 2218–2223 (2005). https://doi.org/10.1007/s10620-005-3038-2
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-3038-2