Abstract
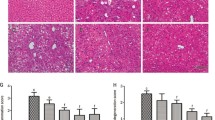
Male Wistar rats were randomized to receive ethanol (2.5 ml/kg by gastric intubation every 8 hr; group I), equal volumes of isocaloric to ethanol sucrose solution (group II), or ethanol and HSS (100 mg/kg intraperitoneally 10 and 16 hr after partial hepatectomy; groups III and IV, respectively) for up to 96 hr after partial hepatectomy, with ethanol administration starting 1 hr prior to partial hepatectomy. Animals were killed at 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48, 60, and 96 hr after partial hepatectomy. The rate of liver regeneration was evaluated by the mitotic index in H&E-stained sections, immunochemical detection of Ki67 nuclear antigen, rate of [3H]thymidine incorporation into hepatic DNA, and liver thymidine kinase enzymatic activity. The biological activity of HSS in groups I and II rats was evaluated using a bioassay. Ethanol administration arrested liver regeneration during the first 32 hr after partial hepatectomy and suppressed HSS activity throughout the period examined. Liver regeneration progressed after 32 hr despite the low levels of HSS activity. HSS administration at 10 and 16 hr reversed liver regeneration arrest induced by ethanol. Acute ethanol administration induces cell cycle arrest during the first 32 hr after partial hepatectomy and suppression of HSS biological activity seems to contribute to this effect. HSS administration reversed the inhibitory effect of ethanol on liver regeneration and caused synchronized entrance of hepatocytes in the S phase of the cell cycle. HSS seems to participate in the network of growth factors controlling the G1/S cell cycle checkpoint.
Similar content being viewed by others
References
Michalopoulos GK, DeFrances MC: Liver regeneration. Science 276(5309):60–66, 1997
Fausto N: Liver regeneration. J Hepatol 32:19–31, 2000
Yamada Y, Kirillova I, Peschon J, Fausto N: Initiation of liver growth by tumor necrosis factor: deficient liver regeneration in mice lacking type I tumor necrosis factor receptor. Proc Natl Acad Sci USA 94:1441–1447, 1997
Cressman DE, Vintermyr OK, Doskeland SO: Liver failure and defective hepatocyte regeneration in interleukine-6-deficient mice. Science 274:1379–1383, 1996
Loyer P, Cariou S, Glaise D, Bilodeau M, Baffert G, Guguen-Guillouzo C: Growth factor dependence of progression through G1 and S phases of adult rat hepatocytes in vitro. J Biol Chem 271:11484–11492, 1996
Albrecht JHM, Hansen LK: Cyclin D1 promotes mitogen-independent cell cycle progression in hepatocytes. Cell Growth Differ 10:397–404, 1999
Nelsen CJ, Rickheim DG, Timchenko NA, Stanley MW, Albrecht JH: Transient expression of cyclin D1 is sufficient to promote hepatocyte replication and liver growth in vivo. Cancer Res 61:8564–8668, 2001
Wada S, Sasaki Y, Horimoto M, Ito T, Ito Y, Tanaka Y, Toyama T, Kasahara A, Hayashi N, Hori M: Involvement of growth factor receptor-bound protein-2 in rat hepatocyte growth. J Gastroenterol Hepatol 13:635–642, 1998
Talarmin H, Rescan C, Cariou S, Glaise D, Zanninelli G, Bilodeau M, Loyer P, Guguen-Guillouzo C, Baffet G: The mitogen-activated protein kinase/extracellular signal-regulated kinase cascade activation is a key signaling pathway involved in the regulation of G1 phase progression in proliferating hepatocytes. Mol Cell Biol 19:6003–6011, 1999
Tsukamoto H, Lu SC: Current concepts in the pathogenesis of alcoholic liver injury. FASEB J 15:1335–1349, 2001
Morales-Gonzales JA, Guittierez-Salinas J, Yanez L, Villagomez-Rico C, Badillo-Romero H, Hernadez-Munoz R: Morphological and biochemical effects of a low ethanol dose on rat liver regeneration: role of route and timing of administration. Dig Dis Sci 44(10):1963–1974, 1999
Diehl AM: Effects of ethanol on liver regeneration. Alcohol Health Res World 17:279–283, 1993
Zhang M, Gong Y, Corbin I, Mellon A, Choy P, Uhanova J, Minuk GY: Light ethanol consumption enhances liver regeneration after partial hepatectomy in the rat. Gastroenterology 19(5):399–401, 2000
Zhang BH, Ho V, Ferrell GC: Specific involvement of Gai2 with epidermal growth factor receptor signaling in rat hepatocytes and the inhibitory effect of chronic ethanol. Biochem Pharmacol 61:1021–1027, 2001
Diehl AM: Effect of ethanol on tumor necrosis factor signaling during liver regenration. Clin Biochem 32(7):571–578, 1999
Akerman PA, Cote PM, Yang SQ, McClain C, Nelson S, Bagby G, Diehl AM: Long-term ethanol consumption alters the hepatic response to the regenerative effects of tumor necrosis factor-alpha. Hepatology 17(6):1066–1073, 1993
Saso, K, Higashi K, Nomura T, Hoshimo M, Ito M, Moehren G, Hoek JB: Inhibitory effect of ethanol on hepatocyte growth factor-induced DNA synthesis and Ca2+ mobilization in rat hepatocytes. Alcohol Clin. Exp. Res 20:330–334, 1996
Koteish A, Yang S, Lin H, Huang J, Diehl AM: Ethanol induces redox-sensitive cell-cycle inhibitors and inhibits liver regeneration after partial hepatectomy. Alcohol Clin Exp Res 26:1710–1718, 2002
Yoshida Y, Komatsu M, Ozeki A, Nango R, Tsukamoto I: Ethanol represses thymidilate synthase and thymidine kinase at mRNA level in regenerating liver after partial hepatectomy. Biochim Biophys Acta 1336:180–186, 1997
Chen J, Bao H, Sawyer S, Kunos G, Gao B: Effects of short and long term ethanol on the activation of signal transducer and activator transcription factor 3 in normal and regenerating liver. Biochem Biophys Res Commun 239:666–669, 1997
Lou G, Zhang M, Minuk GY: Effects of acute ethanol exposure on polyamine and gamma-aminobutiric acid metabolism in the regenerating liver. Alcohol 19:219–227, 1999
Francavilla A, Di Leo A, Polimero L, Gavaler J, Pellici R, Todo S, Kam I, Prelich J, Makowka L, Starzl TE: The effect of hepatic stimulatory substance isolated from regenerating hepatic cytosol and 50,000 and 300,000 subfractions in enhancing survival in experimental acute hepatic failure in rats treated with d-galactozamine. Hepatology 15:485–491, 1992
Fleig WE, Lehmann H, Wagner H, Hoss G, Dischuneit H: Hepatic regenerative stimulator substance in the rabbit: relation to liver regeneration after partial hepatectomy. J Hepatol 3:19–26, 1986
Gupta S, Labreque DR, Shafritz DA: Mitogenic effects of hepatic stimulator substance on cultured nonparenchymal epithelial liver cells. Hepatology 15:485–491, 1992
LaBreque DR: Hepatic stimulator substance: discovery, characteristics and mechanism of action. Dig Dis Sci 39:669–676, 1991
Hagyia M, Francavilla A, Polimero L, Ihara I, Sakai H, Seki T, Simonishi M, Porter MA, Starzl TE: Cloning and sequence analysis of rat Augmenter Of Liver Regeneration (ALR) gene: expression of biologically active ALR and demonstration of tissue distribution. Proc Natl Acad Sci USA 91:8142–8146, 1994
Gandi CR, Kuddus R, Subbobin H, Prelich J, Murase N, Rao AS, Nalesnik MA, Watkins N, Deleo A, Trucco M, Starzl TE: A fresh look on Augmenter of Liver Regeneration in rats. Hepatology 29:1435–1445, 1999
An W, Liu XJ, Lei TG, Dai J, Du GG: Growth induction of hepatic stimulator substance in hepatocytes through its regulation of EGF receptors. Cell Res 9:37–49, 1999
Zhang BH, Gong DZ, Mei MH: Protection of liver regeneration after partial hepatectomy from carbon tetrachloride hepatotoxicity in rats: role of hepatic stimulator substance. J Gastroenterol Hepatol 14:1010–1017, 1999
Kahn D, Svanas G, Eagon P, Makowka L, Podesta L, Chapchap P, Starzl T,Van Thiel D: Effect of an antiandrogenic H2 receptor antagonist on hepatic regeneration in rats. J Lab Clin Med 112:232–239, 1988
Lowry O, Rosenbrough N, Farr A, Randall R: Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275, 1951
Munro H, Fleck A: Recent developments in the measurement of nucleic acids in biological materials. Analyst 91:78–88, 1966
Kyprianidis KG, Mykoniatis MG, Papadimitriou DG, Valsamidou CM: Effect of subtotal pancreatectomy on the rate of liver regenration: the role of Hepatic Stimulator Substance. J Surg Res 62:267–272, 1996
Richards G: Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem 57:369–376, 1974
LaBrecque DR, Bachur NR: Hepatic stimulator substance: physiochemical characteristics and specificity. Am J Physiol 242:G281–G288, 1982
LaBrecque DR, Pesch LA: Preparation and partial characterization of hepatic regenerative stimulator substance (SS) from rat liver. J Physiol 248:273–284, 1975
Gerlach C, Sakkab D, Scholzen T, Dabler R, Alison M, Gerdes J: Ki67 expression during rat liver regeneration after partial hepatectomy. Hepatology 26:573–578, 1997
Liatsos G, Mykoniatis M, Margeli A, Liakos A, Theocharis S: Effect of acute ethanol exposure on the levels of hepatic stimulator substance during liver regeneration: protective function of HSS. Dig Dis Sci 21:1–11, 2003
Gudas G, Fridovich-Keil J, Pardee A: Post-transcriptional control of thymidine kinase messenger RNA accumulation in cells released from G1-G0 blocks. Cell Growth Diff 8:421–430, 1994
Tzirogiannis KN, Panoutsopoulos GI, Demonakou MD, Hereti RI, Alexandropoulou KN, Mykoniatis MG: The hepatoprotective effect of HSS against cadmium-induced apoptosis. Dig Dis Sci 49:1019–1028, 2004
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kondili, V.G., Tzirogiannis, K.N., Androutsos, C.D. et al. The Hepatoprotective Effect of Hepatic Stimulator Substance (HSS) Against Liver Regeneration Arrest Induced by Acute Ethanol Intoxication. Dig Dis Sci 50, 297–307 (2005). https://doi.org/10.1007/s10620-005-1598-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10620-005-1598-9