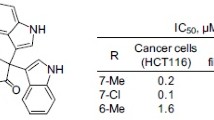
28-Oxo-allobetulone derivatives that were newly synthesized or prepared earlier by modification of ring A (3- and 4-pyridylidene, furfurylidene, azepane, lactam, quinolone, spiroindole, pyrazole, isoxazole, and tetraoxane derivatives) and 3β,19β,28-oleantriol were tested in vitro for cytotoxicity against 60 cell lines of 9 human tumor types. Melanoma SK-MEL-5 and CNS SNB-75 cancer cells died in the presence of 3- or 4-pyridylidene derivatives of 28-oxo-allobetulone. 3β,19β,28-Oleantriol possessed selective cytotoxicity against CNS cancer cell line SNB-75.

Similar content being viewed by others
References
Y. Ye, K. Kinoshita, K. Koyama, K. Takahashi, N. Kondo, and H. Yuasa, J. Nat. Prod., 61, 456 (1998).
W. Dehaen, A. A. Mashentseva, and T. S. Seitembetov, Molecules, 16, 2443 (2011).
T.-S. Li, J.-X. Wang, and X.-J. Zheng, J. Chem. Soc., Perkin Trans. 1, 3957 (1998).
S. Lavoie, A. Pichette, F. X. Garneau, M. Girard, and D. Gaudet, Synth. Commun., 31, 1565 (2001).
O. B. Kazakova, N. I. Medvedeva, D. V. Kazakov, and G. A. Tolstikov, RU Pat. No. 2,402,561, Oct. 27, 2010.
J. A. R. Salvador, R. M. A. Pinto, R. C. Santos, L. C. Roux, A. M. Beja, and J. A. Paixao, Org. Biomol. Chem., 7, 508 (2009).
O. B. Flekhter, N. I. Medvedeva, L. T. Karachurina, L. A. Baltina, F. Z. Galin, F. S. Zarudii, and G. A. Tolstikov, Khim.-farm. Zh., 39 (8), 9 (2005).
E. I. Boreko, N. I. Pavlova, O. V. Savinova, O. B. Flekhter, L. R. Nigmatullina, L. A. Baltina, F. Z. Galin, and G. A. Tolstikov, Belarus Pat. 7809, 2005.
N. V. Galaiko, I. A. Tolmacheva, V. V. Grishko, L. V. Volkova, E. N. Perevozchikova, and S. A. Pestereva, Bioorg. Khim., 36, 556 (2010).
E. F. Khusnutdinova, O. B. Kazakova, A. N. Lobov, O. S. Kukovinets, K. Yu. Suponitsky, C. B. Meyers, and M. N. Prichard, Org. Biomol. Chem., 17, 585 (2019).
S. V. Gein, V. V. Grishko, T. A. Baeva, and I. A. Tolmacheva, Int. J. Pharmacol., 9, 74 (2013).
L. Heller, S. Schwarz, A. Obernauer, and R. Csuk, Bioorg. Med. Chem. Lett., 25, 2654 (2015).
D. Thibeault, C. Gauthier, J. Legault, J. Bouchard, P. Dufour, and A. Pichette, Bioorg. Med. Chem., 15, 6144 (2007).
M. Kvasnica, I. Rudovska, M. Hajduch, and J. Sarek, Monatsh. Chem., 141, 233 (2010).
O. B. Kazakova, I. E. Smirnova, E. F. Khusnutdinova, O. S. Zhukova, L. V. Fetisova, G. N. Apryshko, N. I. Medvedeva, E. Yu. Yamansarov, I. P. Baikova, T. T. Nguyen, and H. Do Thi Thu, Bioorg. Khim., 40, 608 (2014).
D. V. Eroshenko, G. F. Krainova, A. V. Konysheva, M. V. Dmitriev, and V. V. Grishko, Bioorg. Med. Chem. Lett., 28, 3752 (2018).
L. Borkova, S. Gurska, P. Dzubak, R. Burianova, M. Hajduch, J. Sarek, I. Popa, and M. Urban, Eur. J. Med. Chem., 121, 120 (2016).
N. K. Tailor, H. L. Boon, and M. Sharma, Eur. J. Med. Chem., 64, 285 (2013).
D. P. S. Alho, J. A. R. Salvador, M. Cascante, and S. Marin, Molecules, 24, 766 (2019).
A. V. Petrova, E. F. Khusnutdinova, O. B. Kazakova, H. Nguyen Thi Thu, T. Tran Thi Phuong, L. Tran Van, D. Nguyen Thi, and I. E. Smirnova, Vietnam J. Chem., 56, 36 (2018).
E. Yu. Yamansarov, O. B. Kazakova, N. I. Medvedeva, A. N. Lobov, and K. Yu. Suponitsky, Tetrahedron, 73, 4341 (2017).
M. C. Alley, D. A. Scudiero, P. A. Monks, M. L. Hursey, M. J. Czerwinski, D. L. Fine, B. J. Abbott, J. G. Mayo, R. H. Shoemaker, and M. R. Boyd, Cancer Res., 48, 589 (1988).
M. R. Grever, S. A. Schepartz, and B. A. Chabner, Semin. Oncol., 19, 622 (1992).
D. A. Filimonov and V. V. Poroikov, Ross. Khim. Zh., 50, 66 (2006).
D. A. Filimonov and V. V. Poroikov, in: Chemoinformatics Approaches to Virtual Screening, A. Varnek and A. Tropsha (eds.), RSC Publishing, 2008, p. 182.
D. A. Filimonov, A. A. Lagunin, T. A. Gloriozova, A. V. Rudik, D. S. Druzhilovskii, P. V. Pogodin, and V. V. Poroikov, Khim. Geterotsikl. Soedin., 483 (2014).
O. B. Kazakova, N. I. Medvedeva, I. A. Samoilova, I. P. Baikova, G. A. Tolstikov, V. E. Kataev, and V. F. Mironov, Chem. Nat. Compd., 47, 752 (2011).
O. B. Kazakova, E. Yu. Yamansarov, O. S. Kukovinets, and N. I. Medvedeva, Chem. Nat. Compd., 47, 749 (2011).
E. Yu. Yamansarov, O. B. Kazakova, A. N. Lobov, D. V. Kazakov, and K. Yu. Suponitskii, Chem. Nat. Compd., 51, 97 (2015).
O. B. Flekhter, N. I. Medvedeva, G. A. Tolstikov, F. Z. Galin, M. S. Yunusov, H. N. T. Mai, L. V. Tien, I. V. Savinova, E. I. Boreko, L. P. Titov, and I. V. Glukhov, Bioorg. Khim., 35, 253 (2009).
N. I. Medvedeva, O. B. Kazakova, T. V. Lopatina, I. E. Smirnova, G. V. Giniyatullina, I. P. Baikova, and V. E. Kataev, Eur. J. Med. Chem., 143, 464 (2018).
N. Gupta, S. K. Rath, J. Singh, A. Qayum, S. Singh, and P. L. Sangwan, Eur. J. Med. Chem., 135, 517 (2017).
Acknowledgment
The work was performed on State Task Topic No. AAAA-A17-117011910023-2. We thank the National Cancer Institute (NCI) for determining the in vitro antitumor activities of 2–16.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2020, pp. 401–406.
Rights and permissions
About this article
Cite this article
Khusnutdinova, E.F., Smirnova, I.E. & Kazakova, O.B. Synthesis and Cytotoxicity of 28-Oxo-Allobetulone Derivatives. Chem Nat Compd 56, 465–471 (2020). https://doi.org/10.1007/s10600-020-03064-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-020-03064-5