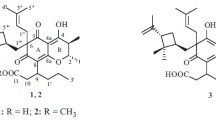
Cyclocondensation of gossypol, the pigment of Gossypium (cotton), and Fischer’s bases produced two new spirocyclic compounds with potentially combined pharmacological and photoswitching activities. The structures and photochemical properties of the synthesized compounds were studied using PMR, IR, and UV spectroscopy.
Similar content being viewed by others
References
M. M. Lerch, M. J. Hansen, G. M. van Dam, W. Szymanski, and B. L. Feringa, Angew. Chem., Int. Ed., 55, 10978 (2016).
W. A. Velema, W. Szymanski, and B. L. Feringa, J. Am. Chem. Soc., 136 (6), 2178 (2014).
W. A. Velema, J. P. van der Berg, M. J. Hansen, W. Szymanski, A. J. M. Driessen, and B. L. Feringa, Nat. Chem., 5, 924 (2013).
H. Bouas-Laurent and H. Durr, Pure Appl. Chem., 73 (4), 639 (2001).
V. I. Minkin, Russ. Chem. Rev., 82 (1), 1 (2013).
R. Klajn, Chem. Soc. Rev., 43 (1), 148 (2014).
B. S. Lukyanov and M. B. Lukyanova, Chem. Heterocycl. Compd., 41, 281 (2005).
N. I. Barum and A. I. Ismailov, Chem. Nat. Compd., 29, 275 (1993).
S. A. Vichkanova and L. V. Goryunova, Antibiotiki, 69, 828 (1968).
V. I. Sumin, A. D. Sakhibov, N. I. Baram, and A. I. Ismailov, Chem. Nat. Compd., 33, 636 (1997).
V. I. Sumin, A. D. Sakhibov, N. I. Baram, and A. I. Ismailov, Chem. Nat. Compd., 35, 165 (1999).
L. Lan, C. Appelman, A. R. Smith, J. Yu, S. Larsen, R. T. Marquez, H. Liu, X. Wu, P. Gao, A. Roy, A. Anbanandam, R. Gowthaman, J. Karanicolas, R. N. De Guzman, S. Rogers, J. Aube, M. Ji, R. S. Cohen, K. L. Neufeld, and L. Xu, Mol. Oncol., 9 (7), 1406 (2015).
B. Wizinger and H. Wenning, Helv. Chim. Acta, 23, 247 (1940).
W. Zhou, Y. T. Li, Y. W. Tang, F. Q. Zhao, X. Q. Song, and E. C. Li, J. Photochem. Photobiol., A, 90 (2), 117 (1995).
S. Aiken, R. J. L. Edgar, C. D. Gabbutt, B. M. Heron, and P. A. Hobson, Dyes Pigm., 149, 92 (2018).
N. A. Voloshin, A. V. Chernyshev, S. O. Bezuglyi, A. V. Metelitsa, E. N. Voloshina, and V. I. Minkin, Russ. Chem. Bull., 57, 151 (2008).
N. E. Gel′man, E. A. Terent′eva, T. M. Shanina, and L. M. Kiparenko, Methods of Quantitative Organic Elemental Analysis [in Russian], Khimiya, Moscow, 1987, 296 pp.
Acknowledgment
The work was performed under a State Research Assignment (Project No. 4.6759.2017/8.9).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 6, November–December, 2018, pp. 918–920.
Rights and permissions
About this article
Cite this article
Malai, V.I., Ozhogin, I.V., Lukyanov, B.S. et al. Novel Spirocyclic Condensation Products of Gossypol and Fischer’s Bases. Chem Nat Compd 54, 1081–1084 (2018). https://doi.org/10.1007/s10600-018-2560-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-018-2560-3