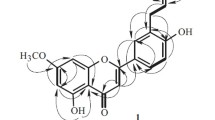
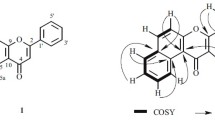
A new flavonoid kaempferol-7,8-diglucoside was isolated for the first time along with two known flavonoid compounds, kaempferol and quercetin, from the methanol extract of Farsetia aegyptia Turra by chromatographic techniques. Two coumarin compounds were isolated and identified from the plant extract. The plant extract contains phenol, catechin, p-hydroxybenzoic acid, gallic acid, pyrogallic acid, p-coumaric acid, and coumarin compounds, which were identified by authentic samples using HPLC. The new compound and alcoholic extract of the plant were evaluated against some bacterial and fungal strains. They showed maximum inhibition against Klebsiella pneumonia. Moderate effects were observed to Aspergillus niger, while the extract did not show activity against Candida albicans.
Similar content being viewed by others
References
I. A. Mitchell-Olds, M. A. Al-Shehbaz, and T. F. Sharbel, Plant Divers. Evol., 8, 119 (2005).
V. Tackolm, Student's Flora of Egypt, 2nd Ed., Cairo University, 1974, p. 183.
L. Boulos, Flora of Egypt, Al-Hadara Publishing, Cairo, Egypt, 1999, p. 203.
A. M. Rizk, The Phytochemistry of the Flora of Qatar. Scientific and Applied Research Center Univ. Qatar, 1986, p. 582.
A. A. Shahat, F. Cuyckens, W. Wang, K. A. Abdel-Shafeek, H. A. Husseiny, S. Apers, S. Van Miert, L. Pieters, A. J. Vlietinck, and M. Claeys, Rapid Commun., Mass Spectrom., 19 (15), 2172 (2005).
I. Tsukasa, Y. Masa-atsu, N. Masayoshi, O. Takashi, Y. Hiroyuki, K. Shuji, O. Hiroshi, and O. Masachika, J. Nat. Prod. Commun., 5 (12), 1903 (2010).
P. K. Agrawal, Carbon-13 NMR of Flavonoids, Elsevier, 1989, p. 581.
F. Cuyckens, A. A. Shahat, H. Van Den Heuvel, K. A. Abdel-Shafeek, M. M. El-Messiry, and El-Nasr. Seif, Eur. J. Mass Spectrom., 9, 409 (2003).
T. P. Tim Cushnie and Andrew J. Lamb, J. Antimicrob. Agents, 26, 343 (2005).
E. Stahl, Thin Layer Chromatography: a Laboratory Handbook, 2nd Ed., George Allen and Unwind London, Springer, Berlin, 1969, p. 1041.
A. S. Awaad, N. M. Sokkr, and G. M. Soliman, Bull. Fac. Pharm. Cairo Univ., 39 (1), 121 (2001).
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer Verlag, New York, Heidelberg, Berlin, 1970, p. 354.
J. B. Harborne, T. J. Mabry, and H. Mabry, The Flavonoids, Chapman and Hall, London, 1975, p. 1204.
H. R. El-Seedi, N. Sata, K. B. G. Torssel, and S. Nishiyama, J. Nat. Prod., 65, 728 (2002).
National Committee for Clinical Laboratory Standards, 1997, NCCLS, Performance standards for antimicrobial disk susceptibility test, 6th Ed., Approved Standard, M2-A6, Wayne, PA.
Y. K. Vaghasiya, R. Nair, M. Soni, S. Balujs, and S. Chanda, J. Serb. Chem. Soc., 69 (12), 991 (2004).
Acknowledgment
The authors are grateful to the Chemistry Department, University of Aberdeen, Scotland, UK, for providing the facilities for instrumental analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Khimiya Prirodnykh Soedinenii, No. 3, May–June, 2013, pp. 370–373.
Rights and permissions
About this article
Cite this article
Atta, E.M., Hashem, A.I. & Eman, R.ES. A novel flavonoid compound from Farsetia aegyptia and its antimicrobial activity. Chem Nat Compd 49, 432–436 (2013). https://doi.org/10.1007/s10600-013-0631-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-013-0631-z