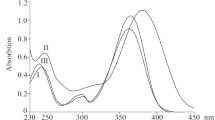
The alkaloid norfluorocurarine was reduced under various conditions to produce the novel bisindoline derivative 2,16-dihydro-19-oxo-C-dihydrotoxiferine and the previously known natural derivatives deoxydihydro-, deoxytetrahydro-, and tetrahydronorfluorocurarine. The structures of the synthesized compounds were identified using IR and UV spectroscopy; of newly produced 2,16-dihydro-19-oxoC-dihydrotoxiferine and deoxydihydronorfluorocurarine, also by x-ray structure analysis. Determination of the absolute configuration of N(α)-methylfluorocurarine chloride dihydrate by x-ray methods enabled the configuration of the 3S,7R,15S asymmetric centers in norfluorocurarine and 2S,3S,7R,15S in deoxydihydronorfluorocurarine to be determined. Also, the absolute configuration of 2,16-dihydro-19-oxoC-dihydrotoxiferine was determined as 2S,3S,7R,15R,16R in one part of the bisindoline and 3'S,7'R,15'S in the other.


Similar content being viewed by others
References
S. Yu. Yunusov and P. Kh. Yuldashev, Dokl. Akad. Nauk UzSSR, 12, 24 (1952).
S. Yu. Yunusov and P. Kh. Yuldashev, Zh. Obshch. Khim., 27, 2015 (1957).
P. Kh. Yuldashev and S. Yu. Yunusov, Uzb. Khim. Zh., No. 1, 44 (1963).
D. Stauffacher, Helv. Chim. Acta, 44, 2006 (1961).
F. A. L. Anet and R. Robinson, J. Chem. Soc., 2253 (1955).
H. Asmus, P. Wasser, H. Schmid, and P. Karrer, Helv. Chim. Acta, 38, 1661 (1955).
D. A. Rakhimov, V. M. Malikov, and S. Yu. Yunusov, Khim. Prir. Soedin., 461 (1969).
Research Progress on Alkaloid-Bearing Plants [in Russian], Fan, Tashkent, 1993, pp. 92–104.
J. Levy, J. Men, and M. M. Janot, Bull. Soc. Chim. Fr., 5, 979 (1960).
M. Fehlmann, H. Koyama, and A. Niggli, Helv. Chim. Acta, 48, 303 (1965).
A. T. McPhail and G. A. Sim, J. Chem. Soc., 1663 (1965).
K. W. Gemmell, J. M. Robertson, G. A. Sim, K. Bernauer, A. Gugg, M. Hesse, H. Schmid, and P. Karrer, Helv. Chim. Acta, 52, 689 (1969).
L. Duport, O. Dideberg, J. Lamotte, K. Kambu, and L. Angelot, Acta Crystallogr. Sect. B: Struct. Crystallogr. Cryst. Chem., 36, 1669 (1980).
K. Bernauer, F. Berlage, W. V. Philipsborn, H. Schmid, and P. Karrer, Helv. Chim. Acta, 41, 2293 (1958).
M. Messerschmidt, S. Scheins, and P. Luger, Acta Crystallogr. Sect. B: Struct. Sci., 61, 115 (2005).
G. M. Sheldrick, Program for Empirical Absorption Correction of Area Detector Data, University of Goettingen, Goettingen, 1996.
Acknowledgment
The work was financed by the Basic Research Program of KKRNT, Rep. Uzb., Grant FA-F3-T045 and the Foundation for Basic Research Support, Acad. Sci., Rep. Uzb., Grant FPFI3910.
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 652–656, September–October, 2010.
Rights and permissions
About this article
Cite this article
Yuldashev, P.K., Tashkhodzhaev, B., Turgunov, K.K. et al. Reduction products of the alkaloid norfluorocurarine. Chem Nat Compd 46, 774–778 (2010). https://doi.org/10.1007/s10600-010-9738-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-010-9738-7