Abstract
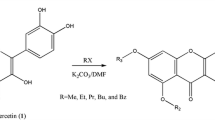
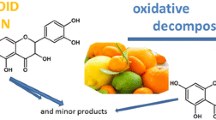
Some of the main oxidation products of quercetin were shown to be compounds formed by oligomerization of the starting flavonoid. Conditions for the preparative synthesis of these compounds were developed. Their structures were established using HPLC-MS and NMR methods. Quercetin oligomers in the natural sample, outer leaves of modified runners of Allium cepa L., were found using chromatographic procedures. The use of quercetin oligomers as indicators of its oxidation was proposed.
Similar content being viewed by others
References
E. M. Cherviakovsky, D. A. Bolibrukh, A. V. Baranovsky, T. M. Vlasova, V. P. Kurchenko, A. A. Gilep, and S._A. Usanov, Biochem. Biophys. Res. Commun., 342, 2, 459 (2006).
C. J. Wallace and A. E. Proudfoot, Biochem. J., 245, 3, 773 (1987).
N. Ohno and M. A. Cusanovich, Biophys. J., 36, 3, 589 (1981).
T. N. Ly, C. Hazama, M. Shimoyamada, H. Ando, K. Kato, and R. Yamauchi, J. Agric. Food Chem., 53, 21, 8183 (2005).
V. Krishnamachari, L. H. Levine, and P. W. Pare, J. Agric. Food Chem., 50, 15, 4357 (2002).
V. Krishnamachari, L. H. Levine, C. Zhou, and P. W. Pare, Chem. Res. Toxicol., 17, 6, 795 (2004).
Y. Hirose, T. Fujita, and M. Nakayama, Chem. Lett., 775 (1999).
A. Gulsen, D. P. Makris, and P. Kefalas, Food Res. Int., 40, 1, 7 (2007).
M. Furusawa, H. Tsuchiya, M. Nagayama, T. Tanaka, K. Nakaya, and M. Iinuma, J. Health Sci., 49, 6, 475 (2003).
M. Furusawa, H. Tsuchiya, M. Nagayama, T. Tanaka, M. Oyama, T. Ito, M. Iinuma, and H. Takeuchi, J. Health Sci., 52, 5, 578 (2006).
P. Schreier and E. Miller, Food Chem., 18, 301 (1985).
F. A. Ramos, Y. Takaishi, M. Shirotori, Y. Kawaguchi, K. Tsuchiya, H. Shibata, T. Higuti, T. Tadokoro, and M. Takeuchi, J. Agric. Food Chem., 54, 10, 3551 (2006).
D. Stajner, N. Milic, J. Canadanovic-Brunet, A. Kapor, M. Stajner, and B. M. Popovic, Phytother. Res., 20, 7, 581 (2006).
U. Takahama and S. Hirota, Plant Cell Physiol., 41, 9, 1021 (2000).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 4, pp. 344–347, July–August, 2008.
Rights and permissions
About this article
Cite this article
Chervyakovsky, E.M., Bolibrukh, D.A., Kurovskii, D.L. et al. Oligomeric oxidation products of the flavonoid quercetin. Chem Nat Compd 44, 427–431 (2008). https://doi.org/10.1007/s10600-008-9092-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10600-008-9092-1