Abstract
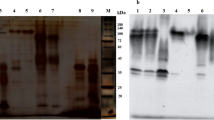
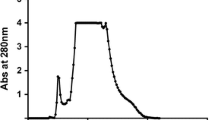
The physicochemical properties of peroxidase isolated from cotton leaves were investigated. The optimal pH value for exhibiting activity was 4.7; temperature, 30°C. The Michaelis constant was 2.3 mM. Cotton-leaf peroxidase has a very high affinity for benzidine.
Similar content being viewed by others
REFERENCES
I. G. Gazaryan (1992) Progress in Science and Technology. Biotechnology VINITI Moscow 4
V. V. Urmantsev (1992) Progress in Science and Technology. Biotechnology VINITI Moscow 54
V. A. Andreeva (1988) Peroxidase Enzyme Nauka Moscow 128
M. Misawa S. M. Martin (1972) Can. J. Biol. 50 1245
C. H. R. Smith R. van Huystee (1989) J. Plant Physiol. 135 391
A. N. Boyarkin (1951) Biokhimiya 16 IssueID7 352
B. J. Davis (1964) Ann. N. Y. Acad. Sci. 121 IssueID2 404
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Prirodnykh Soedinenii, No. 5, pp. 416–418, September–October, 2004.
Rights and permissions
About this article
Cite this article
Akhunov, A.A., Golubenko, Z., Beresneva, Y.V. et al. Physicochemical properties of cotton-leaf peroxidase. Chem Nat Compd 40, 506–509 (2004). https://doi.org/10.1007/s10600-005-0022-1
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10600-005-0022-1