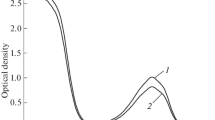
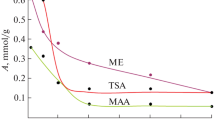
Abstract
It is established that the adsorbability of cinnamic acid from solutions on the surface of highly dispersed silica decreases with an increase in the polarity and electron-donor ability of a solvent. The adsorption process is considerably affected by the hydrophobization of the surface. The adsorption curve passes through a maximum when methyl groups are substituted for 40–55% of the total number of hydroxyls on the silica surface. Probable structures of adsorption complexes are suggested.
Similar content being viewed by others
REFERENCES
Meditsinskaya khimiya i klinicheskoe primenenie dioksida kremniya (Medical Chemistry and Clinical Application of Silica), Chuiko, A.A., Ed., Kiev: Naukova Dumka, 2003.
Pogorelyi, V.K., Barvinchenko, V.N., Lipkovskaja, N.A., et al., in Chemistry, Physics, and Technology of Surfaces, 2001, nos. 4–6, p. 301.
Kurishchuk, K.V., Pentyuk, O.O., and Pogorelii, V.K., Enterosorbent Siliks. Vlastivosti ta klinichne zastosuvannya (Siliks Enterosorbent: Properties and Clinical Application), Kiev: Naukova Dumka, 2000.
Chuiko, A.A., Trakhtenberg, I.M., and Pogorelyi, V.K., Vestn. Farmakol. Farm., 2003, no. 12, p. 16.
Rastitel’nye lekarstvennye sredstva (Vegetable Drugs), Maksyutina, N.P., Ed., Kiev: Zdorov’ya, 1985.
Tertykh, V.A. and Belyakova, L.A., Khimicheskie reaktsii s uchastiem poverkhnosti kremnezema (Chemical Reactions Involving Silica Surface), Kiev: Naukova Dumka, 1991.
Chuiko, O.O., Pogorelii, V.K., Barvinchenko, V.M., et al., Visn. Vinnits. Derzh. Med. Univ., 1999, no. 1, p. 253.
Lipkovskaya, N.A., Pogorelyi, V.K., and Chuiko, A.A., Khim. Farm. Zh., 1997, vol. 31, no.7, p. 44.
Kovtyukhova, N.I. and Pogorelyi, V.K., Ukr. Khim. Zh., 1997, vol. 63, nos.1–2, p. 20.
Zaporozhets, O.A., Lipkovskaja, N.A., Ivanko, L.S., et al., Funct. Mater., 2000, no. 6, p. 2.
Kazakova, O.A., Gun’kov, M.M., Lipkovskaya, N.A., et al., Kolloidn. Zh., 2002, vol. 64, no.4, p. 412.
Gragerov, I.P., Pogorelyi, V.K., and Franchuk, I.F., Vodorodnaya svyaz’ i bystryi protonnyi obmen (Hydrogen Bond and Fast Proton Exchange), Kiev: Naukova Dumka, 1978.
Voyutskii, S.S., Kurs kolloidnoi khimii (Textbook of Colloid Chemistry), Moscow: Khimiya, 1964.
Kiselev, A.V. and Lygin, V.I., Infrakrasnye spektry poverkhnostnykh soedinenii (Infrared Spectra of Surface Compounds), Moscow: Nauka, 1972.
Pal’m, V.A., Osnovy kolichestvennoi teorii organicheskikh reaktsii (Fundamentals of Quantitative Theory of Organic Reactions), Leningrad: Khimiya, 1977.
Author information
Authors and Affiliations
Additional information
__________
Translated from Kolloidnyi Zhurnal, Vol. 67, No. 2, 2005, pp. 201–205.
Original Russian Text Copyright © 2005 by Pogorelyi, Barvinchenko, Pakhlov, Smirnova.
Rights and permissions
About this article
Cite this article
Pogorelyi, V.K., Barvinchenko, V.N., Pakhlov, E.M. et al. The effect of solvent nature on the adsorption interaction between cinnamic acid and silicon dioxide. Colloid J 67, 172–176 (2005). https://doi.org/10.1007/s10595-005-0077-5
Received:
Issue Date:
DOI: https://doi.org/10.1007/s10595-005-0077-5