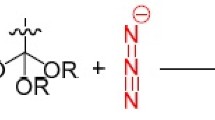Tetrazolic acids (5-substituted 1H-tetrazoles) are of great interest due to their bioisosterism with carboxylic acids, which accounts for plethora of their pharmacological activities and medicinal applications. Many reports deal with the synthesis of tetrazolic acids using different reactions and synthetic methods. This review intends to summarize most of the green approaches available for obtaining these heterocycles. The starting point in the discussion is the application of multicomponent reactions for the construction of structurally diverse products. However, the most commonly used reaction to afford tetrazolic acids is 1,3-dipolar cycloaddition of azide ion to nitriles. Hence, transition from homogeneous catalysis employed in the early examples of this method to heterogeneous catalysis applying recoverable reagents is highlighted. Similarly, the quest for eco-friendly reaction medium is thoroughly described stressing the use of novel systems like ionic liquids and deep eutectic solvents, reactions in aqueous medium, and solvent-free conditions. Further discussion emphasizes the advantages offered by solid-phase synthesis and nonconventional microwave-assisted reactions. Finally, several examples of successful combinations of different green tactics in a same experimental procedure are presented. Thereby, this article aims to provide the main trends for achieving synthesis of tetrazolic acids using environmentally benign procedures reported in the literature while giving perspectives of possible future advances in this topic.


Similar content being viewed by others
References
(a) Myznikov, L. V.; Hrabalek, A.; Koldobskii, G. I. Chem. Heterocycl. Compd.2007, 43, 1. [Khim. Geterotsikl. Soedin.2007, 3.] (b) Dömling, A.; Wang, W.; Wang, K. Chem. Rev.2012, 112, 3083.
(a) Ballatore, C.; Huryn, D. M.; Smith, A. B. ChemMedChem2013, 8, 385. (b) Herr, R. J. Bioorg. Med. Chem.2002, 10, 3379.
(a) Rivera, D. G.; Pérez-Labrada, K.; Lambert, L.; Dörner, S.; Westermann, B.; Wessjohann, L. A. Carbohydr. Res.2012, 359, 102. (b) Cano, P. A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Bioorg. Med. Chem.2014, 22, 1370. (c) Surmiak, E.; Neochoritis, C. G.; Musielak, B.; Twarda-Clapa, A.; Kurpiewska, K.; Dubin, G.; Camacho, C.; Holak, T. A.; Dömling, A. Eur. J. Med. Chem.2017, 126, 384. (d) Claudio-Catalán, M. Á.; Pharande, S. G.; Quezada-Soto, A.; Kishore, K. G.; Rentería-Gómez, A.; Padilla-Vaca, F.; Gámez-Montaño, R. ACS Omega2018, 3, 5177. (e) Méndez, Y.; De Armas, G.; Pérez, I.; Rojas, T.; Valdés-Tresanco, M. E.; Izquierdo, M.; Alonso del Rivero, M.; Álvarez-Ginarte, Y. M.; Valiente, P. A.; Soto, C.; de León, L.; Vasco, A. V.; Scott, W. L.; Westermann, B.; González-Bacerio, J.; Rivera, D. G. Eur. J. Med. Chem.2019, 163, 481.
Neochoritis, C. G.; Zhao, T.; Dömling, A. Chem. Rev.2019, 119, 1970.
(a) Finnegan, W. G.; Henry, R. A.; Lofquist, R. J. Am. Chem. Soc.1958, 80, 3908. (b) Kraus, J. L. Synth. Commun.1986, 16, 827. (c) Koyama, M.; Ohtani, N.; Kai, F.; Moriguchi, I.; Inouye, S. J. Med. Chem.1987, 30, 552.
Anastas, P. T.; Warner, J. C. Green Chemistry: Theory and Practice; Oxford University Press: New York, 1998, p. 30.
(a) Sanderson, K. Nature2011, 469, 18. (b) Clark, J. H. Green Chem.1999, 1, 1.
Mittal, R.; Awasthi, S. K. Synthesis2019, 3765.
Roh, J.; Vávrová, K.; Hrabálek, A. Eur. J. Org. Chem.2012, 6101.
Gunawan, S.; Hulme, C. Org. Biomol. Chem.2013, 11, 6036.
Zhao, T.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Herdtweck, E.; Dömling, A. Chem.–Eur. J.2016, 22, 3009.
Capurro, P.; Moni, L.; Galatini, A.; Mang, C.; Basso, A. Molecules2018, 23, 2758.
(a) Heravi, M. M.; Fazeli, A.; Oskooie, H. A.; Beheshtiha, Y. S; Valizadeh, H. Synlett2012, 2927. (b) Abdollahi-Alibeik, M.; Moaddeli, A. New J. Chem.2015, 39, 2116. (c) Mitra, B.; Mukherjee, S.; Pariyar, G. C.; Ghosh, P. Tetrahedron Lett.2018, 59, 1385.
(a) Bonnamour, J.; Bolm, C. Chem.–Eur. J.2009, 15, 4543. (b) Akhlaghinia, B.; Rezazadeh, S. J. Braz. Chem. Soc.2012, 23, 2197. (c) Patil, D. R.; Deshmukh, M. B.; Dalal, D. S. J. Iran. Chem. Soc.2012, 9, 799. (d) Kumar, S.; Dubey, S.; Saxena, N.; Awasthi, S. K. Tetrahedron Lett.2014, 55, 6034. (e) Bhagat, S. B.; Telvekar, V. N. Synlett2018, 874.
(a) Kantam, M. L.; Kumar, K. B. S.; Sridhar, Ch. Adv. Synth. Catal.2005, 347, 1212. (b) Kantam, M. L.; Balasubrahmanyam, V.; Kumar, K. B. S. Synth. Commun.2006, 36, 1809. (c) Lang, L.; Li, B.; Liu, W.; Jiang, L.; Xu, Z.; Yin, G. Chem. Commun.2010, 46, 448. (d) Sinhamahapatra, A.; Giri, A. K.; Pal, P.; Pahari, S. K.; Bajaj, H. C.; Panda, A. B. J. Mater. Chem.2012, 22, 17227. (e) Lang, L.; Zhou, H.; Xue, M.; Wang, X.; Xu, Z. Mater. Lett.2013, 106, 443.
(a) Nasrollahzadeh, M.; Bayat, Y.; Habibi, D.; Moshaee, S. Tetrahedron Lett.2009, 50, 4435. (b) Eshghi, H.; Seyedi, S. M.; Zarei, E. R. ISRN Org. Chem.2011, 1. (c) Qi, G.; Liu, W.; Bei, Z. Chin. J. Chem.2011, 29, 131.
Rama, V.; Kanagaraj, K.; Pitchumani, K. J. Org. Chem.2011, 76, 9090.
(a) Sajadi, S. M.; Naderi, M.; Babadoust, S. J. Nat. Sci. Res.2011, 1, 2224. (b) Hosseini-Sarvari, M.; Najafvand-Derikvandi, S. C. R. Chim.2014, 17, 1007.
Ai, M.; Lang, L.; Li, B.; Xu, Z. Chem. Lett.2012, 41, 814.
(a) Razavi, N.; Akhlaghinia, B. RSC Adv.2015, 5, 12372. (b) Movaheditabar, P.; Javaherian, M.; Nobakht, V. React. Kinet. Mech. Catal.2017, 122, 217. (c) Sudhakar, K.; Rao, B. P. C.; Kumar, B. P.; Suresh, M.; Ravi, S. Asian J. Chem.2017, 29, 864.
(a) Qi, G.; Dai, Y. Chin. Chem. Lett.2010, 21, 1029. (b) Sreedhar, B.; Kumar, A. S.; Yada, D. Tetrahedron Lett.2011, 52, 3565. (c) Abrishami, F.; Ebrahimikia, M.; Rafiee, F. Appl. Organomet. Chem.2015, 29, 730. (d) Taghavi, F.; Gholizadeh, M.; Saljooghi, A. S.; Ramezani, M. MedChemComm2017, 8, 1953. (e) Jiang, R.; Sun, H.-B.; Li, S.; Zhan, K.; Zhou, J.; Liu, L.; Zhang, K.; Liang, Q.; Chen, Z. Synth. Commun.2018, 48, 2652.
(a) Chermahini, A. N.; Teimouri, A.; Moaddeli, A. Heteroat. Chem.2011, 22, 168. (b) Marvi, O.; Alizadeh, A.; Zarrabi, S. Bull. Korean Chem. Soc.2011, 32, 4001. (c) Rekunge, D. S.; Indalkar, K. S.; Chaturbhuj, G. U. Tetrahedron Lett.2016, 57, 5815.
(a) Du, Z.; Si, C.; Li, Y.; Wang, Y.; Lu, J. Int. J. Mol. Sci.2012, 13, 4696. (b) Nammalwar, B.; Muddala, N. P.; Pitchimani, R.; Bunce, R. A. Molecules2015, 20, 22757.
Shelkar, R.; Singh, A.; Nagarkar, J. Tetrahedron Lett.2013, 54, 106.
Sajadi, S. M.; Mahamb, M. Lett. Org. Chem.2014, 11, 35.
Ghodsinia, S. S. E.; Akhlaghinia, B. RSC Adv.2015, 5, 49849.
(a) Nandre, K. P.; Salunke, J. K.; Nandre, J. P.; Patil, V. S.; Borse, A. U.; Bhosale, S. V. Chin. Chem. Lett.2012, 23, 161. (b) Fortes, M. P.; Bassaco, M. M.; Kaufman, T. S.; Silveira, C. C. RSC Adv.2014, 4, 34519. (c) Artamonova, T.; Zevatskii, Y.; Myznikov, L. Synthesis2014, 81. (d) Ghorbani-Choghamarani, A.; Moradi, P.; Tahmasbi, B. RSC Adv.2016, 6, 56638.
(a) Demko, Z. P.; Sharpless, K. B. J. Org. Chem.2001, 66, 7945. (b) Wang, W.-X.; Cai, H.-L.; Xiong, R.-G. Chin. Chem. Lett.2013, 24, 783. (c) Rad, M. N. S. J. Braz. Chem. Soc.2017, 28, 11. (d) Halder, M.; Islam, M. M.; Singh, P.; Roy, A. S.; Islam, S. M.; Sen, K. ACS Omega2018, 3, 8169. (e) Dofe, V. S.; Sarkate, A. P; Shaikh, Z. M.; Gill, C. H. Heterocycl. Commun.2018, 24, 59.
Himo, F.; Demko, Z. P.; Noodleman, L.; Sharpless, K. B. J. Am. Chem. Soc.2002, 124, 12210.
(a) Kazemnejadi, M.; Sardarian, A. R. RSC Adv.2016, 6, 91999. (b) Esmaeilpour, M.; Sardarian, A. R.; Firouzabadi, H. Appl. Organomet. Chem.2018, 32, e4300. (c) Sardarian, A. R.; Eslahi, H.; Esmaeilpour, M. ChemistrySelect2018, 3, 1499.
(a) Kanakaraju, S.; Prasanna, B.; Chandramouli, G. V. P. J. Chem. Pharm. Res.2012, 4, 2994. (b) Ghumro, S. A.; Alharthy, R. D.; al-Rashida, M.; Ahmed, S.; Malik, M. I.; Hameed, A. ACS Omega2017, 2, 2891. (c) Ghumro, S. A.; Saleem, S.; al-Rashida, M.; Iqbal, N.; Alharthy, R. D.; Ahmed, S.; Moin, S. T.; Hameed, A. RSC Adv.2017, 7, 34197. (d) Xiong, X.; Yi, C.; Liao, X.; Lai, S. Tetrahedron Lett.2019, 60, 402. (e) Padvi, S. A.; Dalal, D. S. Synth. Commun.2017, 47, 779. a Bhosle, M. R.; Shaikh, D. S.; Khillare, L. D.; Deshmukh, A. R.; Mane, R. A. Synth. Commun.2017, 47, 695.
(a) Amantini, D.; Beleggia, R.; Fringuelli, F.; Pizzo, F.; Vaccaro, L. J. Org. Chem.2004, 69, 2896. (b) Rostamizadeh, S.; Ghaieni, H.; Aryan, R.; Amani, A. Chin. Chem. Lett.2009, 20, 1311. (c) Xie, A.; Cao, M.; Feng, L.; Dong, W. J. Chem. Res.2013, 37, 665. (d) Rahman, M.; Roy, A.; Ghosh, M.; Mitra, S.; Majee, A.; Hajra, A. RSC Adv.2014, 4, 6116. (e) Jahanshahi, R.; Akhlaghinia, B. RSC Adv.2015, 5, 104087.
(a) Roh, J.; Artamonova, T. V.; Vávrová, K.; Koldobskii, G. I.; Hrabálek, A. Synthesis2009, 2175. (b) Coca, A.; Feinn, L.; Dudley, J. Synth. Commun.2015, 45, 1023. (c) Padmaja, R. D.; Meena, D. R.; Maiti, B.; Chanda, K. Res. Chem. Intermed.2017, 43, 7365.
(a) Matthews, D. P; Green, J. E.; Shuker, A. J. J. Comb. Chem.2000, 2, 19. (b) Gunn, S. J.; Baker, A.; Bertram, R. D; Warriner, S. L. Synlett2007, 2643. (c) Cousaert, N.; Willand, N.; Gesquière, J.-C.; Tartar, A.; Déprez, B.; Deprez-Poulain, R. Tetrahedron Lett.2008, 49, 2743.
Kivrakidou, O.; Bräse, S.; Hülshorst, F.; Griebenow, N. Org. Lett.2004, 6, 1143.
Morales, F. E.; Garay, H. E.; Muñoz, D. F.; Augusto, Y. E.; Otero-González, A. J.; Reyes-Acosta, O.; Rivera, D. G. Org. Lett.2015, 17, 2728.
(a) Tisseh, Z. N.; Dabiri, M.; Nobahar, M.; Khavasi, H. R.; Bazgir, A. Tetrahedron2012, 68, 1769. (b) Ahmed, N.; Siddiqui, Z. N. RSC Adv.2015, 5, 16707. (c) Akbarzadeh, P.; Koukabi, N.; Kolvari, E. Res. Chem. Intermed.2019, 45, 1009. (d) Safaei-Ghomi, J.; Paymard-Samani, S.; Zahedi, S.; Shahbazi-Alavi, H. Z. Naturforsch. B: J. Chem. Sci.2015, 70, 819. (e) Khalafi-Nezhad, A.; Mohammadi, S. RSC Adv.2013, 3, 4362.
(a) Zarghani, M.; Akhlaghinia, B. RSC Adv.2016, 6, 31850. (b) Azadi, G.; Ghorbani-Choghamarani, A.; Shiri, L. Transit. Met. Chem.2017, 42, 131. (c) Darabi, M.; Tamoradi, T.; Ghadermazi, M.; Ghorbani-Choghamarani, A. Transit. Met. Chem.2017, 42, 703. (d) Tamoradi, T.; Mehraban-Esfandiari, B.; Ghadermazi, M.; Ghorbani-Choghamarani, A. Res. Chem. Intermed.2018, 44, 1363. (e) Reddivari, C. K. R.; Devineni, S. R.; Venkateshwarulu, J. K. M; Baki, V. B.; Chippada, A. R.; Wudayagiri, R.; Venkata, R. R. Y.; Chamarthi, N. R. Eur. J. Chem.2017, 8, 66. (f) Mehraban, J. A.; Azizi, K.; Jalali, M. S; Heydari, A. ChemistrySelect2018, 3, 116. (g) Salahshournia, B.; Hamadi, H.; Nobakht, V. Appl. Organomet. Chem.2018, 32, e4416. a Rezaei, F.; Amrollahi, M. A.; Khalifeh, R. Inorg. Chim. Acta2019, 489, 8.
Gerardo M. Ojeda-Carralero acknowledges VLIR-UOS for the financial support in frame of Flemish-Cuba cooperation project (CU2018TEA458A101).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Published in Khimiya Geterotsiklicheskikh Soedinenii, 2020, 56(4), 408–421
Rights and permissions
About this article
Cite this article
Ojeda-Carralero, G.M., Coro, J. & Valdés-Palacios, A. Green alternatives for the synthesis of tetrazolic acids. Chem Heterocycl Comp 56, 408–421 (2020). https://doi.org/10.1007/s10593-020-02676-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-020-02676-7