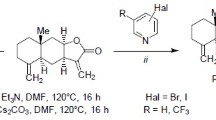
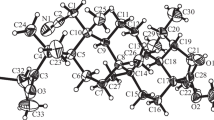
The Heck reaction of methylidene lactones of guaiane type, arglabin and ludartin, with aryl halides occurs with the formation of the respective (E)- and (Z)-13-arylidene-substituted derivatives and the respective endocyclic isomer, the ratio of which depends on the nature of methylidenelactone and the reaction conditions. The arylation of ludartin occurred with a lower yield of the target products and was accompanied by the formation of chamazulene. The interaction of grosheimin with aryl halides under Heck reaction led to the exocyclic products with (E)- and (Z)-configuration, with the latter as the major isomers. The structure of two compounds was established by X-ray structural analysis.

Similar content being viewed by others
References
Patrushev, S. S.; Shakirov, M. M.; Shults, E. E. Chem. Heterocycl. Compd. 2016, 52, 165. [Khim. Geterotsikl. Soedin. 2016, 52, 165.]
(a) Fraga, B. M. Nat. Prod. Rep. 2013, 30, 1226. (b) Schall, A.; Reiser, O. Eur. J. Org. Chem. 2008, 2353. (c) Santana, A.; Molinillo, J. M. G.; Macías, F. A. Eur. J. Org. Chem. 2015, 2093.
Ding, Y.-H.; Fan, H.-X.; Long, J.; Zhang, Q.; Chen, Y. Bioorg. Med. Chem. Lett. 2013, 23, 6087.
(a) Adekenov, S. M.; Mukhametzhanov, M. N.; Kagarlitskii, A. D.; Kupriyanov, A. N. Chem. Nat. Compd. 1982, 18, 623. [Khim. Prirod. Soedin. 1982, 655.] (b) Appendino, G.; Gariboldi, P.; Menichini, F. Fitoterapia 1991, 62, 275. (c) Adekenov, S. M. Chem. Nat. Compd. 2013, 49, 158. [Khim. Prirod. Soedin. 2013, 140.]
(a) Jalmakhanbetova, R. I.; Adekenov, S. M. Chem. Nat. Compd. 2007, 43, 347. [Khim. Prirod. Soedin. 2007, 287.] (b) Lone S. H., Bhat, K. A.; Naseer, S.; Rather, R. A.; Khuroo, M. A.; Tasduq, S. A. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2013, 940, 135. (c) Zeng, Y.-T.; Jiang, J.-M.; Lao, H.-Y.; Guo, J.-W.; Lun, Y.-N.; Yang, M. Mol. Med. Rep. 2015, 11, 2234.
(a) Adekenov, S. M. Eurasian Chem.-Technol. J. 2013, 15, 163. (b) Shimoda, H.; Ninomiya, K.; Nishida, N.; Yoshino, T.; Morikawa, T.; Matsuda, H.; Yoshikawa, M. Bioorg. Med. Chem. Lett. 2003, 13, 223. (c) Li, X.; Qian, P.; Liu, Z.; Zhao, Y.; Xu, G.; Tao, D.; Zhao, Q.; Sun, H. Heterocycles 2005, 65, 287.
(a) Jalmahanbetova, R. I.; Rakhimova, B. B.; Raldugin, V. A.; Bagryanskaya, I. Y.; Gatilov, Y. V.; Shakirov, M. M.; Kulyjasov, A. T.; Adekenov, S. M.; Tolstikov, G. A. Russ. Chem. Bull., Int. Ed. 2003, 52, 748. [Izv. Akad. Nauk, Ser. Khim. 2003, 715.] (b) Jalmakhanbetova, R. I.; Atazhanova, G. A.; Raldugin, V. A.; Bagryanskaya, I. Y.; Gatilov, Y. V.; Shakirov, M. M.; Adekenov, S. M. Chem. Nat. Compd. 2007, 43, 548. [Khim. Prirod. Soedin. 2007, 450.] (c) Csuk, R.; Heinold, A.; Siewert, B.; Schwarz, S.; Barthel, A.; Kluge, R.; Ströhl, D. Arch. Pharm. (Weinheim, Ger.) 2012, 345, 215. (d) Lone, S. H.; Bhat, K. A.; Khuroo, M. A. Chem.-Biol. Interact. 2015, 240, 180.
(a) Lone, S. H.; Bhat, K. A.; Shakeel-u-Rehman; Majeed, R.; Hamid, A.; Khuroo M. A. Bioorg. Med. Chem. Lett. 2013, 23, 4931. (b) Lone, S. H.; Bhat, K. A.; Majeed, R.; Hamid, A.; Khuroo M. A. Bioorg. Med. Chem. Lett. 2014, 24, 1047. (c) Lone, S. H.; Bhat, K. A. Tetrahedron Lett. 2015, 56, 1908.
(a) Ivasenko, S. A.; Kulyyasov, A. T.; Adekenov, S. M. Chem. Nat. Compd. 2003, 39, 601. [Khim. Prirod. Soedin. 2003, 497.] (b) Ivasenko, S. A.; Dzhalmakhanbetova, R. I.; Kulyyasov, A. T.; Kurmankulov, N. B.; Adekenov, S. M. Chem. Nat. Compd. 2004, 40, 387. [Khim. Prirod. Soedin. 2004, 316.] (c) Petkevich, S. K.; Kishkentaeva, A. S.; Ryazantsev, O. G.; Dikusar, E. A.; Zhukovskaya, N. A.; Kletskov, A. V.; Kozlov, N. G.; Atazhanova, G. A.; Adekenov, S. M.; Potkin, V. I.; Pashkevich, S. G.; Denisov, A. A.; Kulchitskii, V. A. Russ. J. Org. Chem. 2014, 50, 1351. [Zh. Org. Khim. 2014, 50, 1366.]
(a) Shults, E. E.; Tolstikov, G. A. Russ. Chem. Bull., Int. Ed. 2013, 62, 605. [Izv. Akad. Nauk, Ser. Khim. 2013, 605.] (b) Shults, E. E. Eurasian Chem.-Technol. J. 2013, 15, 175. (c) Patrushev, S. S.; Shakirov, M. M.; Rybalova, T. V.; Shults, E. E. Chem. Heterocycl. Compd. 2014, 50, 1063. [Khim. Geterotsikl. Soedin. 2014, 155.] (d) Mukusheva, G. K.; Lipeeva, A. V.; Zhanymkhanova, P. Zh.; Shults, E. E.; Gatilov, Yu. V.; Shakirov, M. M.; Adekenov, S. M. Chem. Heterocycl. Compd. 2015, 51, 146. [Khim. Geterotsikl. Soedin. 2015, 51, 146.]
(a) Jeffery, T. Tetrahedron 1996, 52, 10113. (b) Bönnemann, H.; Brinkmann, R.; Köppler, R.; Neiteler, P.; Richter, J. Adv. Mater. (Weinheim, Ger.) 1992, 4, 804.
(a) Shults, E. E.; Belovodskii, A. V.; Shakirov, M. M.; Gatilov, Yu. V.; Pokrovskii, A. G.; Pokrovskii, M. A.; Tolstikov, G. A. Chem. Nat. Compd. 2012, 48, 238. [Khim. Prirod. Soedin. 2012, 215.] (b) Esenbaeva, A. E.; Shults, E. E.; Gatilov, Yu. V.; Shakirov, M. M.; Patrushev, S. S.; Atazhanova, G. A.; Kenesheva, A. B.; Adekenov, S. M. Chem. Nat. Compd. 2013, 49, 875. [Khim. Prirod. Soedin. 2013, 752.]
(a) Macías, F. A.; Viñolo, V. M. I.; Fronczek, F. R.; Massanet, G. M.; Molinillo, J. M. G. Tetrahedron 2006, 62, 7747. (b) Fazilova, A. S.; Turdibekov, K. M. Khim. Zh. Kazakhstan 2005, 230.
Belovodskii, A. V.; Shults, E. E.; Shakirov, M. M.; Bagryanskaya, I. Yu.; Gatilov, Yu. V.; Tolstikov, G. A. Russ. J. Org. Chem. 2010, 46, 1719. [Zh. Org. Khim. 2010, 1710.]
Shults, E. E.; Belovodskii, A. V.; Shakirov, M. M.; Tolstikov, G. A. Russ. Chem. Bull., Int. Ed. 2012, 61, 1975. [Izv. Akad. Nauk, Ser. Khim. 2012, 1959.]
Dzhemilev, U. M.; Popod'ko, N. R.; Kozlova, E. V. Metal Complex Catalysis in Organic Synthesis [in Russian]; Khimiya: Moscow, 1999, p. 104.
The work was performed with financial support from the Russian Science Foundation (project No. 14-13-00822). The authors gratefully acknowledge the contribution by the Collective Chemical Service Center of Siberian Branch of the Russian Academy of Sciences for the spectral and analytical studies.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2016, 52(10), 788–796
* For Communication 9, see1.
Rights and permissions
About this article
Cite this article
Kihkentayeva, A.S., Shults, E.E., Gatilov, Y.V. et al. Synthetic Transformations of Sesquiterpene Lactones 10*. Synthesis of 13-Arylguaianolides. Chem Heterocycl Comp 52, 788–796 (2016). https://doi.org/10.1007/s10593-016-1967-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-016-1967-7