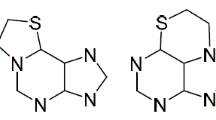
A series of novel N-(purin-6-yl)- and N-(2-aminopurin-6-yl) derivatives of heterocyclic amines was synthesized. It was found that preferred preparative method for N-(purin-6-yl) derivatives of heterocyclic amines is interaction of 6-chloropurine with heterocyclic amines without any catalyst; whereas N-(2-aminopurin-6-yl) derivatives were obtained in the moderate-to-high yields while heating 2-amino-6-chloropurine and heterocyclic amines in water in the presence of sulfuric acid.

Similar content being viewed by others
Notes
Eletskaya, B. Z.; Konstantinova, I. D.; Paramonov, A. S.; Esipov, R. S.; Gruzdev, D. A.; Vigorov, A. Yu.; Levit, G. L.; Miroshnikov, A. I.; Krasnov, V. P.; Charushin, V. N. Mendeleev Commun. 2015, in press.
Eletskaya, B. Z.; Konstantinova, I. D.; Paramonov, A. S.; Esipov, R. S.; Gruzdev, D. A.; Vigorov, A. Yu.; Levit, G. L.; Miroshnikov, A. I.;
Krasnov, V. P.; Charushin, V. N. Mendeleev Commun. 2015, in press
Eletskaya, B. Z.; Konstantinova, I. D.; Paramonov, A. S.; Esipov, R. S.; Gruzdev, D. A.; Vigorov, A. Yu.; Levit, G. L.; Miroshnikov, A. I.; Krasnov, V. P.; Charushin, V. N. Mendeleev Commun. 2015, in press.
Eletskaya, B. Z.; Konstantinova, I. D.; Paramonov, A. S.; Esipov, R. S.; Gruzdev, D. A.; Vigorov, A. Yu.; Levit, G. L.; Miroshnikov, A. I.; Krasnov, V. P.; Charushin, V. N. Mendeleev Commun. 2015, in press.
References
Rosemeyer, H. Chem. Biodiversity 2004, 1, 361.
Legraverend, M.; Grierson, D. S. Bioorg. Med. Chem. 2006, 14, 3987.
Gray, N. S.; Wodicka, L.; Thunnissen, A.-M. W. H.; Norman, T. C.; Kwon, S.; Espinoza, F. H.; Morgan, D. O.; Barnes, G.; LeClerc, S.; Meijer, L.; Kim, S.-H.; Lockhart, D. J.; Schultz, P. G. Science 1998, 281, 533.
Chang, Y.-T.; Gray, N. S.; Rosania, G. R.; Sutherlin, D. P.; Kwon, S.; Norman, T. C.; Sarohia, R.; Leost, M.; Meijer, L.; Schultz, P. G. Chem. Biol. 1999, 6, 361.
Canela, M.-D.; Liekens, S.; Camarasa, M.-J.; Priego, E. M.; Pérez-Pérez, M.-J. Eur. J. Med. Chem. 2014, 87, 421.
Berger, J.; Flippin, L. A.; Greenhouse, R.; Jaime-Figueroa, S.; Liu, Y.; Miller, A. K.; Putman, D. G.; Weinhardt, K. K.; Zhao, S.-H. US Patent 5958934; Chem. Abstr. 1999, 131, 243281.
Ciszewski, L.; Waykole, L.; Prashad, M.; Repić, O. Org. Process Res. Dev. 2006, 10, 799.
Chen, G.; Cushing, T. D.; Fisher, B.; He, X.; Li, K.; Li, Z.; McGee, L. R.; Pattaropong, V.; Faulder, P.; Seganish, J. L.; Shin, Y. WO Patent 2009158011; Chem. Abstr. 2009, 152, 119631.
Xie, L.; Wang, X.; Lee, K.-H. WO Patent 2013178075; Chem. Abstr. 2013, 160, 7466.
Kochergin, P. M.; Persanova, L. V.; Aleksandrova, E. V. Chem. Heterocycl. Compd. 2000, 36, 455. [Khim. Geterotsikl. Soedin. 2000, 529.]
Weigele, M.; Shakespeare, W.; Sawyer, T. K.; Sundaramoorthi, R.; Bohacek, R.; Wang, Y.; Metcalf III, C. A. WO Patent 2001044260; Chem. Abstr. 2001, 135, 46049.
Estep, K. G.; Josef, K. A.; Bacon, E. R.; Carabateas, P. M.; Rumney IV, S.; Pilling, G. M.; Krafte, D. S.; Volberg, W. A.; Dillon, K.; Dugrenier, N.; Briggs, G. M.; Caniff, P. C.; Gorczyca, W. P.; Stankus, G. P.; Ezrin, A. M. J. Med. Chem. 1995, 38, 2582.
Bordon Pallier, F.; Haesslein, J. L. FR Patent 2851248; Chem. Abstr. 2004, 141, 206968.
Novosjolova, I.; Bizdēna, E.; Turks, M. Eur. J. Org. Chem. 2015, 3629.
Demange, L.; Oumata, N.; Quinton, J.; Bouaziz, S.; Lozach, O.; Meijer, L.; Galons, H. Heterocycles 2008, 75, 1735.
Houzé, S.; Hoang, N.-T.; Lozach, O.; Le Bras, J.; Meijer, L.; Galons, H.; Demange, L. Molecules 2014, 19, 15237.
Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, 2012.
Wade, L. G., Jr. Organic Chemistry, 8th ed.; Prentice Hall: Upper Saddle River, New Jersey, 2013.
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Inc.: Hoboken, New Jersey, 2010.
Banks, K. J. Am. Chem. Soc. 1944, 66, 1127.
Banks, K. J. Am. Chem. Soc. 1944, 66, 1131.
Chapman, N. B.; Rees, C. W. J. Chem. Soc. 1954, 1190.
Liu, J.; Robins, M. J. J. Am. Chem. Soc. 2007, 129, 5962.
Whitfield, H. J.; Griffin, R. J.; Hardcastle, I. R.; Henderson, A.; Meneyrol, J.; Mesguiche, V.; Sayle, K. L.; Golding, B. T. Chem. Commun. 2003, 2802.
Wong, C.; Griffin, R. J.; Hardcastle, I. R.; Northen, J. S.; Wang, L.-Z.; Golding, B. T. Org. Biomol. Chem. 2010, 8, 2457.
Carbain, B.; Coxon, C. R.; Lebraud, H.; Elliott, K. J.; Matheson, C. J.; Meschini, E.; Roberts, A. R.; Turner, D. M.; Wong, C.; Cano, C.; Griffin, R. J.; Hardcastle, I. R.; Golding, B. T. Chem.–Eur. J. 2014, 20, 2311.
Carter, C. E. J. Biol. Chem. 1956, 223, 139.
Garrett, E. R.; Mehta, P. J. J. Am. Chem. Soc. 1972, 94, 8532.
Wong, J. L.; Fuchs, D. S. J. Chem. Soc., Perkin Trans 1 1974, 1284.
Fujii, T.; Saito, T.; Hisata, H.; Shinbo, K. Chem. Pharm. Bull. 1990, 38, 3326.
Lönnberg, H.; Lehikoinen, P. Nucl. Acids Res. 1982, 10, 4339.
Lönnberg, H.; Heikkinen, E. Acta Chem. Scand. Ser. B 1984, 38, 673.
Ahlbrecht, H.; Düber, E. O.; Epsztajn, J.; Marcinkowski, R. M. K. Tetrahedron 1984, 40, 1157.
Oldham, W.; Johns, I. B. J. Am. Chem. Soc. 1939, 61, 3289.
Gruzdev, D. A.; Chulakov, E. N.; Levit, G. L.; Ezhikova, M. A.; Kodess, M. I.; Krasnov, V. P. Tetrahedron: Asymmetry 2013, 24, 1240.
Hayakawa, I.; Hiramitsu, T.; Tanaka, Y. EU Patent 0047005; Chem. Abstr. 1982, 97, 55821b.
Charushin, V. N.; Gorbunov, E. B.; Rusinov, G. L.; Likholobov, V. A.; Rodionov, V. A. RU Patent 2434005; Chem. Abstr. 2011, 155, 683753.
Charushin, V. N.; Krasnov, V. P.; Levit, G. L.; Korolyova, M. A.; Kodess, M. I.; Chupakhin, O. N.; Kim, M. H.; Lee, H. S.; Park, Y. J.; Kim, K.-C. Tetrahedron: Asymmetry 1999, 10, 2691.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gruzdev, D.A., Musiyak, V.V., Chulakov, E.N. et al. Synthesis of purine and 2-aminopurine conjugates bearing the fragments of heterocyclic amines at position 6. Chem Heterocycl Comp 51, 738–744 (2015). https://doi.org/10.1007/s10593-015-1767-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-015-1767-5