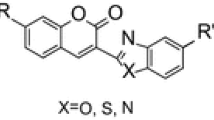
Methods of synthesis and functionalization of coumarins using metal complex and organic catalysis are considered in the review.
Similar content being viewed by others
References
A. Vogel, Gilbert’s Ann. Phys., 64, 161 (1820).
R. O’Kennedy and R. D. Thornes (editors), Coumarins, Biology, Applications and Mode of Action, John Wiley and Sons, Chichester (1997), p. 348.
J. R. S. Hoult and M. Paya, Gen. Pharmacol., 27, 713 (1996).
H. Kobayashi, M. Ogawa, R. Alford, P. L. Choyke, and Y. Urano, Chem. Rev., 110, 2620 (2010).
R. D. H. Murray, J. Mendez, and S. A. Brown, The Natural Coumarins, Occurrence and Biochemistry, John Wiley and Sons, Chichester (1982).
R. D. H. Murray, Prog. Chem. Org. Nat. Prod., 58, 83 (1991).
R. D. H. Murray, Natl. Prod. Rep., 12, 477 (1995).
A. Estévez-Braun and A. G. Gonzalez, Nat. Prod. Rep., 14, 465 (1997).
V. M. Malikov and A. I. Saidkhodzhaev, Chem. Nat. Compd., 34, 202 (1998).
S. Sardari, S. Nishibe, and M. Daneshtalab, Stud. Nat. Prod. Chem., 23, 335 (2000).
F. Borges, F. Roleira, N. Milhazes, L. Santana, and E. Uriarte, Curr. Med. Chem., 12, 887 (2005).
M. E. Riveiro, N. De Kimpe, A. Moglioni, R. Vazquez, F. Monczor, C. Shayo, and C. Davio, Curr. Med. Chem., 17, 1325 (2010).
M. M. Garazd, Y. L. Garazd, and V. P. Khilya, Chem. Nat. Compd., 41, 245 (2005).
G. Volmajer, R. Toplak, I. Leban, and A. Majcen Le Marechal, Tetrahedron, 61, 7012 (2005).
V. K. Ahluwalia, D. Singh, and R. P. Singh, Monatsh. Chem., 116, 869 (1985).
E. Rizzi, S. Dallavalle, L. Merlini, G. Pratesi, and F. Zunino, Synth. Commun., 36, 1117 (2006).
K. Sato and T. Amakasu, J. Org. Chem., 33, 2446 (1968).
J. R. Bantick and J. L. Suschitzky, J. Heterocycl. Chem., 18, 679 (1981).
B. M. Trost and F. D. Toste, J. Am. Chem. Soc., 118, 6305 (1996).
C. Jia, W. Lu, J. Oyamada, T. Kitamura, K. Matsuda, M. Irie, Y. Fujiwara, J. Am. Chem. Soc., 122, 7252 (2000).
C. Jia, D. Piao, J. Oyamada, W. Lu, T. Kitamura, and Y. Fujiwara, Science, 287, 1992 (2000).
S. Aoki, J. Oyamada, and T. Kitamura, Bull. Chem. Soc. Japan, 78, 468 (2005).
S. Aoki, C. Amamoto, J. Oyamada, and T. Kitamura, Tetrahedron, 61, 9291 (2005).
C. Jia, D. Piao, T. Kitamura, and Y. Fujiwara, J. Org. Chem., 65, 7516 (2000).
S. J. Pastine, S. W. Youn, and D. Sames, Org. Lett., 5, 1055 (2003).
K. H. Park, I. G. Jung, and Y. K. Chung, Synlett, 2541 (2004).
Y. Yamamoto and N. Kirai, Org. Lett., 10, 5513 (2008).
X. Han and X. Lu, Org. Lett., 12, 108 (2010).
M. Catellani, G. P. Chiusoli, G. Marzolini, and E. Rossi, J. Organomet. Chem., 525, 65 (1996).
T. Meng, Y. Zou, O. Khorev, Y. Jin, H. Zhou, Y. Zhang, D. Hu, L. Ma, X. Wang, and J. Shen, Adv. Synth. Cat., 353, 918 (2011).
D. V. Kadnikov and R. C. Larock, J. Org. Chem., 68, 9423 (2003).
D. V. Kadnikov and R. C. Larock, Org. Lett. 23, 3643 (2000).
A. K. Chatterjee, F. D. Toste, S. D. Goldberg, and R. H. Grubbs, Pure Appl. Chem., 75, 421 (2003).
T. N. Van, S. Debenedetti, and N. De Kimpe, Tetrahedron Lett., 44, 4199 (2003).
U. Gross, P. J. Gross, M. Shi, and S. Bräse, Synlett, 635 (2011).
G. M. Boland, D. M. X. Donnelly, J.-P. Finet, and M. D. Rea, J. Chem. Soc., Perkin Trans 1, 2591 (1996).
C. Bailly, C. Bal, P. Barbier, S. Combes, J.-P Finet, M.-P. Hildebrand, V. Peyrot, and N. Wattez, J. Med. Chem., 46, 5437 (2003).
D. M. X. Donnelly, J.-P. Finet, P. J. Guiry, and M. D. Rea, Synth. Commun., 29, 2719 (1999).
I. P. Beletskaya, O. G. Ganina, A. V. Tsvetkov, A. Yu. Fedorov, and J.-P. Finet, Synlett, 2797 (2004).
O. G. Ganina, E. Daras, V. Bourgarel-Rey, V. Peyrot, A. N. Andresyuk, J.-P. Finet, A. Yu. Fedorov, I. P. Beletskaya, and S. Combes, Bioorg. Med. Chem., 16, 8806 (2998).
M. I. Naumov, A. V. Nuchev, N. S. Sitnikov, Yu. B. Malysheva, A. S. Shavyrin, I. P. Beletskaya, A. E. Gavryushin, S. Combes, and A. Yu. Fedorov, Synthesis, 1673 (2009).
N. S. Sitnikov, A. S. Shavyrin, G. K. Fukin, I. P. Beletskaya, S. Combes, and A. Yu. Fedorov, Izv. Akad. Nauk, Ser. Khim., 3, 612 (2010). [Russ. Chem. Bull., 59, 626 (2010)].
S. Combes, P. Barbier, S. Douillard, A. McLeer-Florin, V. Bourgarel-Rey, J.-T. Pierson, A. Yu. Fedorov, J.-P. Finet, J. Boutonnat, and V. Peyrot, J. Med. Chem., 54, 3153 (2011).
Y. Luo and J. Wu, Tetrahedron Lett., 50, 2103 (2009).
A. N. Selikhov, Yu. B. Malysheva, A. V. Nyuchev, N. S. Sitnikov, E. A. Sharonova, A. S. Shavyrin, S. Combes, and A. Yu. Fedorov, Izv. Akad. Nauk, Ser. Khim., 1968 (2011).
H. Guo, E. Herdtweck, and T. Bach, Angew. Chem., Int. Ed., 49, 7782 (2010).
A. V. Nyuchev, E. A. Sharonova, N. A. Lenshina, A. S. Shavyrin, M. A. Lopatin, I. V. Balalaeva, I. P. Beletskaya, and A. Yu. Fedorov, Tetrahedron Lett., 52, 4196 (2011).
Y. Luo and J. Wu, Tetrahedron, 65, 6810 (2009).
J. A. Key, S. Koh, Q. K. Timerghazin, A. Brown, and C. W. Cairo, Dyes Pigm., 82, 196 (2009).
S. Valente and G. Kirsch, Tetrahedron Lett., 52, 3429 (2011).
S. Martins, P. S. Branco, M. C. de la Torre, M. A. Sierra, and A. Pereira, Synlett, 2918 (2010).
A. R. Das, A. Medda, and R. Singha, Tetrahedron Lett., 51, 1099 (2010).
D. Audisio, S. Messaoudi, J.-F. Peyrat, J.-D. Brion, and M. Alami, Tetrahedron Lett., 48, 6928 (2007).
O. Ganina, A. Yu. Fedorov, and I. P. Beletskaya, Synthesis, 3689 (2009).
L. Zhang, T. Meng, R. Fan, and J. Wu, J. Org. Chem., 72, 7279 (2007).
S. H. Wunderlich and P. Knochel, Angew. Chem., Int. Ed., 46, 7685 (2007).
R. D. Rieke and S.-H. Kim, Tetrahedron Lett., 52, 3094 (2011).
F. Gosselin, R. A. Britton, I. W. Davies, S. J. Dolman, D. Gauvreau, R. S. Hoerrner, G. Hughes, J. Janey, S. Lau, C. Molinaro, C. Nadeau, P. D. O'Shea, M. Palucki, and R. Sidler, J. Org. Chem., 75, 4154 (2010).
K. Sivakumar, F. Xie, B. M. Cash, S. Long, H. N. Barnhill, and Q. Wang, Org. Lett., 6, 4603 (2004).
X.-P. He, Z. Song, Z.-Z. Wang, X.-X. Shi, K. Chen, and G.-R. Chen, Tetrahedron, 67, 3343 (2011).
F. Seela, V. R. Sirivolu, and P. Chittepu, Bioconjugate Chem., 19, 211 (2008).
Y. Zhou, K. Liu, J.-Y. Li, Y. Fang, T.-C. Zhao, and C. Yao, Org. Lett., 13, 1290 (2011).
J. C. Morris, J. C. McMurtrie, S. E. Bottle, and K. E. Fairfull-Smith, J. Org. Chem., 76, 4964 (2011).
J. Lu, M. Shi, and M. S. Shoichet, Bioconjugate Chem., 20, 87 (2009).
P. Chittepu, V. R. Sirivolu, and F. Seela, Bioorg. Med. Chem., 16, 8427 (2008).
N. Anand, N. Jaiswal, S. K. Pandey, A. K. Srivastava, and R. P. Tripathi, Carbohydr. Res., 346, 16 (2011).
A. V. Nyuchev, E. A. Sharonova, N. A. Lenshina, A. S. Shavyrin, M. A. Lopatin, I. V. Balalaeva, I. P. Beletskaya, and A. Yu. Fedorov, Tetrahedron Lett., 52, 4196 (2011).
X. Zhu, A. Lin, Y. Shi, J. Guo, C. Zhu, and Y. Cheng, Org. Lett., 13, 4382 (2011).
H.-M. Yang, L. Li, K.-Z. Jiang, J.-X. Jiang, G.-Q. Lai, and L.-W. Xu, Tetrahedron, 66, 9708 (2010).
K. C. Majumdar, R. K. Nandi, S. Samanta, and B. Chattopadhyay, Synthesis, 6, 985 (2010).
K. C. Majumdar, B. Chattopadhyay, and S. Nath, Tetrahedron Lett., 49, 1609 (2008).
L. Chen and M.-H. Xu, Adv. Synth. Catal., 351, 2005 (2009).
R. V. Rozhkov and R. C. Larock, Org. Lett., 5, 797 (2003).
L.-P. Fan, P. Li, X.-S. Li, D.-C. Xu, M.-M. Ge, W.-D. Zhu, and J.-W. Xie, J. Org. Chem., 75, 8716 (2010).
L. Chen, Y. Li, and M.-H. Xu, Org. Biomol. Chem., 8, 3073 (2010).
L. Tang, Y. Pang, Q. Yan, L. Shi, J. Huang, Y. Du, and K. Zhao, J. Org. Chem., 76, 2744 (2011).
J. Padwal, W. Lewis, and C. J. Moody, Synlett, 514 (2010).
M. Rueping, E. Merino, and E. Sugiono, Adv. Synth. Catal., 350, 2127 (2008).
Y. Gao, Q. Ren, L. Wang, and J. Wang, Chem. Eur. J., 16, 13068 (2010).
H. Zhao, A. C. Donnelly, B. R. Kusuma, G. E. L. Brandt, D. Brown, R. A. Rajewski, G. Vielhauer, J. Holzbeierlein, M. S. Cohen, and B. S. J. Blagg, J. Med. Chem., 54, 3839 (2011).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 1, pp. 175-186, January, 2012.
Rights and permissions
About this article
Cite this article
Fedorov, A.Y., Nyuchev, A.V. & Beletskaya, I.P. Catalytic methods of creation and functionalization of the coumarin skeleton. Chem Heterocycl Comp 48, 166–178 (2012). https://doi.org/10.1007/s10593-012-0980-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-012-0980-8